Antibacterial Resistance Patterns Among Common Infections in a Tertiary Care Hospital in Saudi Arabia - Cureus
Background
The rapid emergence of antibiotic-resistant bacteria threatens the control of infectious diseases by reducing treatment effectiveness, prolonging illness duration, and increasing healthcare costs. This study aimed to identify the common rate of bacterial resistance against antibacterial agents in tertiary healthcare providers in Saudi Arabia.
Methodology
This retrospective cross-sectional observational study was conducted from May 2016 to December 2019 on 1,151 urinary tract infection (UTI) and respiratory tract infection (RTI) positive cultures collected from participants aged 15 years or older who received antibiotic treatment. The obtained variables included age, gender, diagnosis, antibiotic type, specimen source, culture results, and sensitivity test results.
Results
The most common bacteria in UTI were Escherichia coli (46.7%), followed by Klebsiella pneumoniae (30.5%). Moreover, E. coli was most resistant to ampicillin (56.4%), followed by ceftriaxone (33.8%). Among the respiratory cultures, the most frequently isolated pathogen was Pseudomonas aeruginosa (28.5%), followed by K. pneumoniae (17.6%). The 162 respiratory P. aeruginosa isolates were most resistant to piperacillin/tazobactam (51.9%), followed by ciprofloxacin (25%) and ampicillin (10.6%).
Conclusion
High levels of antibiotic resistance were observed in both Gram-negative and Gram-positive bacteria. This indicates a need for better implementation of antibacterial stewardship and increased awareness of appropriate antibiotic use to limit the rapid spread of antibacterial resistance.
Introduction
The introduction of antibiotics to the medical field was one of the greatest discoveries in the history of medicine. When penicillin was introduced in the 1940s by Alexander Fleming, a new era of therapeutic medicine was established [1]. The outcomes of bacterial infections saw a great turnaround as fatal and severe infections became easily treatable. However, the efficiency of antibiotics has decreased as many available antibiotics are no longer effective along with the emergence of antibiotic-resistant (ABR) strains. Importantly, antibiotic resistance discovery is related to resistance detection in clinical samples; however, the resistance might be discovered earlier according to the observation from laboratory samples.
Globally, it is estimated that ABR infections are responsible for approximately 700,000 deaths per year [2,3]. If no preventative actions are taken, it is predicted that infections caused by ABR bacteria will have a mortality rate exceeding that of cancer and become the most common cause of death by the end of 2050 [2,3]. According to the Centers for Disease Control and Prevention (CDC), approximately 35,900 deaths out of 2,868,700 ABR cases are expected to be reported annually in the United States [2]. In 2012, Aly et al. investigated antimicrobial resistance in 37,295 bacterial isolates collected from different hospitals in the Gulf region. Within this sample, the most prevalent microorganism was Escherichia coli, followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter, Clostridium difficile, and Enterococcus [4]. In addition, a study conducted by the Saudi national surveillance on Gram-positive cocci revealed that 32% of S. aureus belonged to MRSA, 33% of S. pneumoniae were resistant to penicillin G, and 26% of S. pneumoniae were resistant to erythromycin [3]. In the western region of Saudi Arabia, Alam et al. reported bacterial resistance to trimethoprim/sulfamethoxazole (48.6%), ampicillin (49.3%), piperacillin (59.3%), and methicillin (50.3%) [5].
Bacteria have the unique ability to lower or eliminate the antimicrobial efficacy of drugs and chemical agents [6]. This may occur through natural resistance (e.g., β-lactamase production) or acquired resistance [7,8]. Acquired bacterial resistance may occur through four mechanisms. One of these mechanisms is the production of enzymes that modify or inhibit antibiotic action [7-9]. Another mechanism is through changes in the permeability of bacterial cell walls [7]. Bacteria can also acquire resistance through disruptions in protein synthesis [7], alterations in metabolic pathways, or genetic mutations [8]. Finally, bacteria can acquire resistance from the transferred copy of the plasmid (R-plasmid genes) of a previously resistant bacteria [7-9].
The development of antibiotic resistance appears to be inevitable [10]. However, the overuse and misuse of antibiotics are accelerating this process [11]. The misuse of antibiotics is a complex problem driven by several factors related to patients, healthcare providers, and institutional healthcare regulations [12]. Public knowledge, awareness, and attitudes regarding antibiotic use are strong determinants of antibiotic misuse [13]. In a systematic review conducted by Alhomoud et al. in 2017 and demonstrated the use of antibiotics in the Middle East, the overall prevalence of participants who used antibiotics as self-prescription ranged from 19% to 82% [14]. The highest prevalence of self-prescription antibiotics was reported in Yemen and Oman followed by Saudi Arabia [14]. Access to antibiotics without a prescription and gaps in knowledge and safe practices regarding antibiotics' use (e.g., keeping leftover antibiotics from an uncompleted course for future use and sharing antibiotics with others) were among the reported reasons for self-medication with antibiotics [14]. Furthermore, prescribers' knowledge and attitudes regarding antibiotic use and resistance have been reported to determine the quality of antibiotic prescriptions [15]. One core problem underlying improper antibiotic prescription is the lack of sufficient diagnostic tests to rapidly identify pathogens and their antibiotic susceptibility profiles [16]. Another proven risk factor for antibiotic resistance is travel, specifically during the Hajj season, when the acquisition and transmission of infectious diseases (including those caused by ABR bacteria) are common occurrences [17].
The topic of antibiotic resistance has been approached from many perspectives for a wide variety of clinical and social practices and implications. However, the present research specifically aimed to assess the prevalence of ABR infections in Ministry of National Guard-Health Affairs (MNGHA), Jeddah, Saudi Arabia. In a study conducted in the western region of Saudi Arabia, pneumonia was the most prevalent infectious disease reported in patients aged 26-45 years [18]. Additionally, pneumonia and urinary tract infections (UTIs) were the most prevalent forms of infectious diseases among female patients [18]. In the central region of Saudi Arabia, respiratory tract infections (RTIs) and UTIs have been found to be the most frequent complaints encountered in emergency departments [19]. The availability of updated epidemiological data from a given region or community is important not only for the optimization of empirical therapies but also for the implementation of an effective antimicrobial stewardship program in hospitals [20].
Materials & Methods
Selection criteria
An observational cross-sectional quantitative study (with non-probability convenience sampling) was conducted in the MNGHA, Jeddah, Saudi Arabia. For this study, patients were selected according to the following criteria: male and female Saudi inpatients and outpatients aged 15 years or older who had received antibiotic treatments prior to the initiation of the study for UTIs and/or RTIs. This sample excluded the oncology department, patients infected with tuberculosis (TB), and patients infected with human immunodeficiency virus (HIV).
Sample size calculation
The sample size was calculated using Raosoft® software (Raosoft Inc., Seattle, United States). Approximately 231,000 patients received antibiotic treatments in MNGHA, Jeddah, between May 2016 and December 2019. At a 95% confidence level, an estimated 59.1% prevalence of ABR patients, and a 5% margin of error, the required minimum sample size was estimated at 371 samples. All patients who met the sample criteria from May 2016 to December 2019 were included in the study.
Data were obtained from the BESTCare system (ezCaretech, Torrance, California, United States) using a data collection sheet. The collected numerical variables included age and date of diagnosis, and the collected categorical/nominal variables included gender, hospital setting, diagnosis, antibiotic type, specimen source, culture results, and sensitivity test results.
Data analysis
Parametric and non-parametric approaches were used to describe the numerical data (age and date of diagnosis). Percentages were used to describe the categorical variables (gender, hospital setting, diagnosis, antibiotic type, specimen source, type of organism, and sensitivity test results). Chi-square or Fisher exact test was used to compare categorical data, while t-test and ANOVA were used to make comparisons between categorical and numerical variables. A p-value of less than 0.05 was statistically significant. All data were analyzed using IBM SPSS Statistics for Windows, Version 20.0 (Released 2011; IBM Corp., Armonk, New York, United States).
Ethical approval
The study was carried out in line with the Helsinki protocol and ethical approval from the Institutional Review Board of King Abdullah International Medical Research Centre, Jeddah, Saudi Arabia, was duly acquired prior to conducting this study (approval number: SP20/050/J, dated April 22, 2020). No names and Identities (IDs) were collected from the participants, and the data were stored within 64‑bit encrypted software on the work computer of the primary investigator that was not liable to be breached by nonauthorized persons.
Results
A total of 1,151 isolates were obtained from the BESTCare system in MNGHA, Jeddah, Saudi Arabia, between May 2016 and December 2019. These samples were categorized into age groups. Overall, 52.7% (n = 607) of these samples were collected from female patients, and 78.2% (n = 900) and 21.8% (n = 251) were collected from inpatients and outpatients, respectively. Data regarding patient demographics, hospital settings, and specimen types are displayed in Table 1.
Regarding specimen sources, 49.3% (n = 568) of the samples were obtained from respiratory specimens, 50.7% (n = 583) were obtained from urine specimens, and the sources of seven specimens were not documented; thus, the total number of specimens was 1,144 (Table 2).
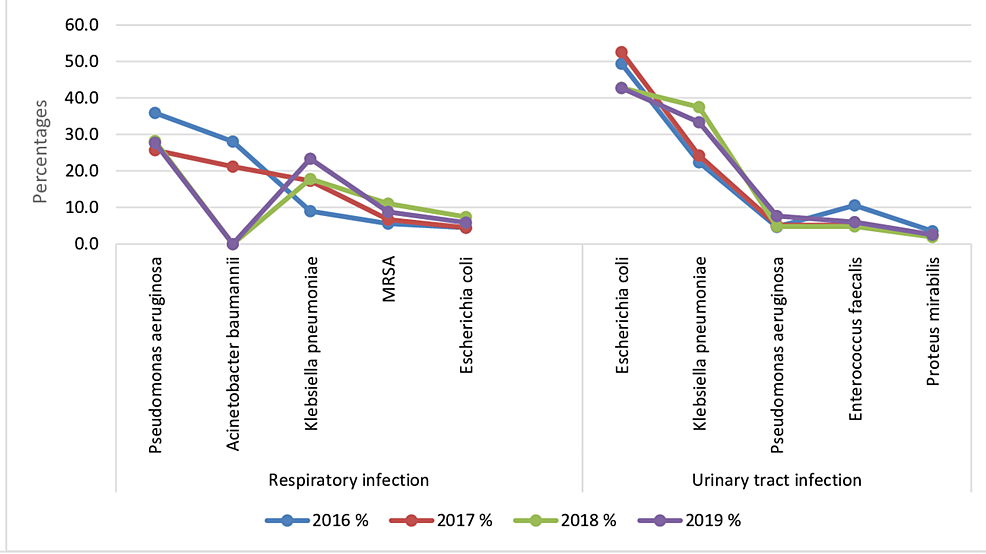
The top 10 most common causative agents of UTIs and RTIs were E. coli (26.4%; n = 304), K. pneumoniae (24.2%; n = 278), P. aeruginosa (16.9%; n = 194), Acinetobacter baumannii (8.4%; n = 97), MRSA (3.6%; n = 42), Enterococcus faecalis (3%; n = 35), S. aureus (2.9%; n = 33), Proteus mirabilis (2.1%; n = 24), Haemophilus influenzae (2%; n = 23), and S. pneumoniae (1.8%; n = 21) (Figure 1, Table 3).
The most common microbial causative agent of UTIs was E. coli (46.7%; n = 272), followed by K. pneumoniae (30.5%; n = 178), E. faecalis (6%; n = 35), P. aeruginosa (5.5%; n = 32), and P. mirabilis (2.2%; n = 13) (Table 4).
Furthermore, E. coli was most resistant to ampicillin (56.4%), followed by ceftriaxone (33.8%), ciprofloxacin (3.8%), amoxicillin (2.6%), and trimethoprim/sulfamethoxazole (1.7%; p = 0.014). Similarly, K. pneumoniae was most resistant to ampicillin (69.7%), followed by ceftriaxone (23.9%), amoxicillin/clavulanate (2.8%), amoxicillin (1.7%), and ciprofloxacin (0.6%; p < 0.001). Meanwhile, E. faecalis was most resistant to ciprofloxacin (28.6%), followed by ampicillin (21.4%), erythromycin and clindamycin (14.3%), and vancomycin (7.1%; p = 0.619). The P. aeruginosa isolates were most resistant to piperacillin/tazobactam (53.8%), followed by ciprofloxacin and ampicillin/sulbactam (15.4%) and cefazolin and trimethoprim/sulfamethoxazole (7.7%; p = 0.023). Finally, P. mirabilis was most resistant to ampicillin (53.8%), followed by ciprofloxacin (23.1%), nitrofurantoin (15.4%), and trimethoprim/sulfamethoxazole (7.7%; p = 0.860). The complete results are illustrated in Table 5 and Table 6.
Regarding the isolates from respiratory sources, the most frequently isolated pathogen was P. aeruginosa (28.5%), followed by K. pneumoniae (17.6%), A. baunmannii (15.1%), MRSA (7.2%), and E. coli (5.6%) (Table 7).
Regarding the 162 respiratory P. aeruginosa isolates, most (51.9%) were resistant to piperacillin/tazobactam, followed by ciprofloxacin (25%), ampicillin (10.6%), ampicillin/sulbactam (3.8%), and meropenem (2.9%; p < 0.001). Meanwhile, K. pneumoniae was most resistant to ampicillin (82.7%), followed by ceftriaxone (9.2%), piperacillin/tazobactam (7.1%), and amoxicillin (1%; p = 0.153). Finally, the A. baunmannii isolates were most resistant to piperacillin/tazobactam (52.6%; p = 0.520). Table 8 and Table 9 give details of respiratory infection bacterial resistence.
Comments
Post a Comment