Nitric oxide is a host cue for Salmonella Typhimurium systemic ... - Nature.com
Abstract
Nitric oxide (NO) is produced as an innate immune response against microbial infections. Salmonella Typhimurium (S. Typhimurium), the major causative pathogen of human gastroenteritis, induces more severe systemic disease in mice. However, host factors contributing to the difference in species-related virulence are unknown. Here, we report that host NO production promotes S. Typhimurium replication in mouse macrophages at the early infection stage by activating Salmonella pathogenicity island-2 (SPI-2). The NO signaling-induced SPI-2 activation is mediated by Fnr and PhoP/Q two-component system. NO significantly induced fnr transcription, while Fnr directly activated phoP/Q transcription. Mouse infection assays revealed a NO-dependent increase in bacterial burden in systemic organs during the initial days of infection, indicating an early contribution of host NO to virulence. This study reveals a host signaling-mediated virulence activation pathway in S. Typhimurium that contributes significantly to its systemic infection in mice, providing further insights into Salmonella pathogenesis and host–pathogen interaction.
Introduction
Innate immune responses, including the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), represent the first line of host defense against microbial infections by limiting replication and spread of invading pathogens1. On the other hand, microbial pathogens have developed strategies to antagonize innate immune responses and some even exploit those responses as signals to promote their own virulence2,3. The interaction between host innate immune functions and pathogen virulence mechanisms largely determines the outcome of most infections4.
Salmonella is an important intracellular pathogen that causes a range of diseases in humans and animals. Infections by Salmonella represent a considerable burden in both developing and developed countries with more than 110 million cases in humans reported every year5,6. Depending on serovar/host combinations, Salmonella infections are generally either localized to the gastrointestinal tract, resulting in mild gastroenteritis, or disseminated further to extraintestinal sites, causing severe systemic diseases such as typhoid fever7,8,9. Salmonella enterica serovar Typhimurium (S. Typhimurium) is the main causative agent for human gastroenteritis but can induce a typhoid-like systemic infection in mice10,11. How S. Typhimurium interacts differently with hosts, leading to the severe virulence in mice is not clear.
The ability to survive and replicate in host macrophages is essential to the systemic infection of S. Typhimurium12,13. Accordingly, S. Typhimurium replicates to high numbers in mouse macrophages, in a manner that requires a specific type III secretion system (T3SS) encoded by Salmonella pathogenicity island-2 (SPI-2)14. Expression of SPI-2 genes under the control of SPI-2-encoded SsrA (the integral membrane cognate sensor)/SsrB (the response regulator) two-component regulatory system (TCS), the master regulator of all SPI-2 operons, is induced upon phagocytosis by various host cues, including cation deprivation, phosphate starvation, and phagosome acidification generated by innate immune responses15,16. The SPI-2-encoded T3SS injects effector proteins, both encoded within and outside of SPI-2, into host cells to induce the formation of a specialized membrane-bound compartment called the Salmonella-containing vacuole (SCV) within which S. Typhimurium survives and replicates17,18,19. SPI-2 mutants of S. Typhimurium showed intracellular proliferation defect in primary mouse peritoneal macrophages (PMs) and mouse macrophage cell lines such as RAW264.7 and J774A.120,21,22, and were found to be highly attenuated in the systemic infection mouse model infected orally, intraperitoneally (i.p.), or intravenously20,22,23,24. Although the expression of SPI-2 is also induced in human macrophages, which are less permissive for S. Typhimurium replication, the induction level is much lower than that observed in mouse macrophages25,26, indicating the presence of host-specific cues for SPI-2 activation in mouse macrophages.
Nitric oxide (NO), the RNS prototype, is produced as an innate immune response against microbial infection by inducible nitric oxide synthase (iNOS), which converts arginine into citrulline and NO27,28. While ROS is responsible for the initial killing of pathogens, RNS reportedly inhibits S. Typhimurium replication in infected organs and within infected macrophages at later stages29,30. However, NO by itself poses only weak antibacterial activity31. Noticeably, NO production was detected in mouse macrophages (5–20 μM) infected with S. Typhimurium32,33. However, human macrophages, which are less permissive for S. Typhimurium replication, generally show low levels of iNOS expression and produce negligible amounts of NO34,35,36,37. This suggests a positive correlation between host NO production and SPI-2 expression and S. Typhimurium replication. Interestingly, S. Typhimurium avoids direct contact with both ROS and RNS by residing within the SCV33,38. However, as NO is freely diffusible into the SCV, it is curious whether host NO production contributes to severe S. Typhimurium virulence in mice.
TCSs are signaling pathways via that bacteria sense and respond to environmental or cellular parameters and thus adapt to the changing conditions39. The PhoP/Q TCS, a master regulator of S. Typhimurium virulence functions, generally activates SPI-2 via both ssrB at the transcriptional level and ssrA at the posttranscriptional level by sensing multiple host cues40. Intramacrophage signal sensing by PhoP/Q is critical for SPI-2-mediated virulence of S. Typhimurium in mice40,41, as S. Typhimurium mutants lacking the PhoP/Q system are highly attenuated for virulence in mice and unable to survive within macrophages12,42. Whether PhoP/Q senses or responds to host NO production has not been investigated.
Fnr is a well-known global anaerobic regulator and contributes to the virulence of many bacterial pathogens that encounter changes in O2 availability43,44,45. Fnr is also required for S. Typhimurium systemic infection, as the fnr mutant was completely attenuated in both orally and i.p.-infected mice and the lack of fnr resulted in a dramatic reduction in the ability of S. Typhimurium to replicate in PMs46. A microarray-based transcriptome analysis reveals that Fnr positively regulates many invasion-associated virulence genes, but has no effect on SPI-2, in S. Typhimurium46. As this study was conducted using S. Typhimurium grown in Luria–Bertani (LB) to log phase, which is a non-SPI-2-inducing condition16, whether Fnr contributes to SPI-2 induction in host macrophages is uncertain. Fnr is also known as a NO-responsive regulator in bacteria44. Binding to NO inactivates Fnr by reacting with its [4Fe-4S]2+ cluster, thereby impacting the expression of Fnr-regulated genes45. Whether NO affects the transcription level of fnr is not known.
In this study, the contribution of host NO production to S. Typhimurium systemic infection in mice was investigated using mouse infection assays, gentamicin protection assays, RNA sequencing (RNA-seq), SPI-2 gene (ssrA) promoter substitution analyses, and many other molecular techniques. We found that host NO production promotes S. Typhimurium replication in mouse macrophages by activating SPI-2, and increases the bacterial burden in the liver and spleen of infected mice at early infection stages. Further investigations revealed that the NO-signaling-induced SPI-2 activation is mediated by Fnr and PhoP/Q. Thus, this study reveals that NO produced by the innate immune system is a host cue for S. Typhimurium virulence activation in mice, providing further insight into Salmonella pathogenesis.
Results
Host NO levels correlate positively with S. Typhimurium replication and SPI-2 expression levels in mouse macrophages
Previous studies reported that NO is produced in mouse but almost not in human macrophages in response to S. Typhimurium infection32,33,47, and this was confirmed here. Up to 20 μM NO (within the range reported previously) was detected in mouse RAW264.7 while less than 1 μM was detected in human U937 macrophage cells during the 24 h infection period with S. Typhimurium wild-type strain ATCC 14028 s (Fig. 1a). Negligible NO production was also detected in human THP-1 cells (Supplementary Fig. 1a). The difference in NO production between RAW264.7 and U937 cells with LPS treatment was further confirmed (Supplementary Fig. 1b). Providing the different virulence to mice (high) and humans (low), S. Typhimurium replicated better in mouse macrophages than in human macrophages48. In agreement, intracellular bacterial burden of S. Typhimurium was much higher in RAW264.7 cells than that in U937 cells as indicated by gentamicin protection assays (Fig. 1b). Differences in NO production and intracellular bacterial burden were further confirmed in primary mouse bone-marrow-derived macrophages (BMDMs) and in primary human peripheral blood mononuclear cells (PBMCs) (Supplementary Fig. 1c, d). The increase in bacterial burden was less in BMDMs (3.2-fold at 16 h) than in RAW264.7 cells (17.4-fold at 16 h), and this was likely due to the absence of inflammasome activation in RAW264.7 cells, which do not express the inflammasome adapter protein ASC.
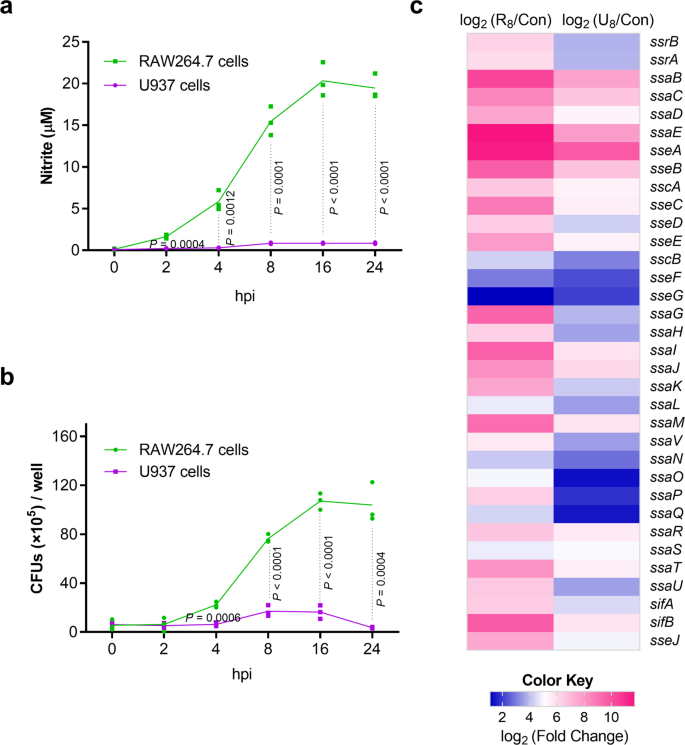
a Nitrite production by RAW264.7 and U937 cells infected with S. Typhimurium wild-type strain ATCC 14028 s. RAW264.7 cells and U937 cells were infected at a MOI of 10, and nitrite levels in the supernatant were measured using Griess assays at the indicated time points (n = 3 independent experiments). b Bacteria burden of S. Typhimurium wild-type in RAW264.7 and U937 cells. Bacterial CFU (×105) /well (y axis) and time after addition of gentamicin (x axis) are indicated (n = 3 independent experiments). c Higher expression of S. Typhimurium SPI-2 genes in RAW264.7 cells than that in U937 cells. RAW264.7 cells and U937 cells were infected with S. Typhimurium wild-type for 8 h (MOI = 10), cell were then lysed and the intraceullar bacteria were collected for RNA extraction and RNA-seq (R8: RAW264.7 8 h; U8, U937 8 h). RNA extracted from bacteria in the RPMI-1640 medium was used as control (Con) (n = 3 independent experiments). The data are taken from Supplementary Table 1. All data are presented as mean ± SD. P values were determined using two-way ANOVA (a, b). hpi hours post-infection. Source data are included in Supplementary Data 1.
As SPI-2 is required for S. Typhimurium replication in mouse macrophages49, the induction of SPI-2 expression in RAW264.7 cells was confirmed by RNA-seq profiling (Fig. 1c and Supplementary Table 1) and quantitative real-time PCR (qRT-PCR) analysis of seven SPI-2 genes (ssrA, ssrB, sipC, ssaE, sscA, sifA, ssaV; Supplementary Fig. 1e). The expression of SPI-2 in U937 cells was also induced, but the level was significantly lower than that in RAW264.7 cells (Fig. 1c and Supplementary Table 1). Therefore, there is a positive correlation between host NO production and SPI-2 expression and replication levels of S. Typhimurium in mouse macrophages, implying that NO produced by the innate immune response might contribute to S. Typhimurium virulence during systemic infection in mice.
Lack of host NO reduced S. Typhimurium SPI-2 gene expression and bacterial replication in mouse macrophages, and reduced bacterial burden in mouse systemic organs at early infection stages
To investigate whether NO production in mouse macrophages contributes to S. Typhimurium virulence, RAW264.7 cells were treated with L-NMMA, a competitive inhibitor of iNOS, 2 h prior to infection to inhibit NO production, and the expression of SPI-2 genes in treated and non-treated cells was assessed at various time points during the infection period. Compared to untreated cells, the expression levels of ssaG, a representative SPI-2 gene, were significantly reduced in L-NMMA-treated RAW264.7 cells at each time point during the 24 h infection period as determined by qRT-PCR analysis (Fig. 2a). The same results were obtained using primary PMs from C57BL/6 mice (Fig. 2b). The addition of L-NMMA did not affect bacterial uptake by macrophages, as the treated and untreated RAW264.7 cells and PMs contained comparable number of intracellular bacteria 15 min post-infection (Supplementary Fig. 2a). Furthermore, the expression levels of ssaG were also significantly decreased in iNOS−/− PMs (iNOS-deficient macrophages from iNOS-immunodeficient C57BL/6 mice), that are unable to produce NO, compared to that in wild-type C57BL/6 mouse PMs (Fig. 2c). The supplementation of NO increased ssaG expression in iNOS−/− PMs (Fig. 2d). Thus, NO production in mouse macrophages promotes SPI-2 expression in S. Typhimurium.
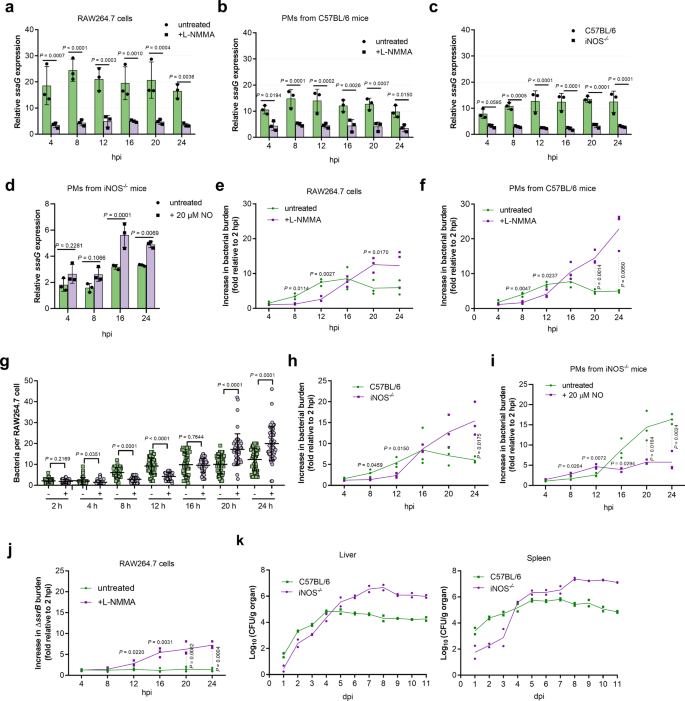
a–c qRT-PCR analysis of S. Typhimurium ssaG mRNA levels in RAW264.7 cells (a), peritoneal macrophages (PMs) from C57BL/6 mice (b), and PMs from iNOS−/− mice (c) at the indicated time points in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). d qRT-PCR analysis of ssaG expression in PMs from iNOS−/− mice at 4, 8, 16, and 24 h post-infection (hpi) in the presence or absence of 20 µM NO (n = 3 independent experiments). e, f, h Increase in bacterial burden of S. Typhimurium wild-type in RAW264.7 cells (e), PMs from C57BL/6 mice (f), and PMs from iNOS−/− mice (h) at the indicated time points in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). g Number of intracellular bacteria per RAW264.7 cells (n = 75 cells per group pooled from three independent experiments). The number of bacteria per cell were counted in random fields. −, untreated; +, addition of 50 μM L-NMMA. Representative immunofluorescence images (8, 12, and 24 hpi) are provided in Supplementary Fig. 2b. i Increase in bacterial burden of S. Typhimurium wild-type in PMs from iNOS−/− mice at indicated time points in the presence or absence of 20 µM NO (n = 3 independent experiments). j Replication of S. Typhimurium ssrB mutant in RAW264.7 cells at the indicated time points in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). k Bacterial counts recovered from the liver and spleen of wild-type and iNOS−/− C57BL/6 mice intraperitoneally (i.p.) infected with S. Typhimurium wild-type at the indicated time points post-infection, n = 2 mice each day. All data are presented as mean ± SD. P values were determined using two-way ANOVA (a–f, h–k) or two-tailed unpaired Student's t test (g). hpi hours post-infection, dpi days post-infection. Source data are included in Supplementary Data 1.
As SPI-2 is required for S. Typhimurium replication in mouse macrophages, whether host NO production contributes to S. Typhimurium replication in mouse macrophages was investigated. Replication assays were carried out in untreated and L-NMMA-treated RAW264.7 cells and C57BL/6 mouse PMs. Compared to that in untreated cells, the intracellular burden of S. Typhimurium was significantly reduced in L-NMMA-treated cells at early infection stages (first 4 to 12 h) (Fig. 2e, f). Immunofluorescence enumeration further confirmed the presence of lower bacterial number in L-NMMA-treated RAW264.7 cells 12 h post-infection (Fig. 2g and Supplementary Fig. 2b). However, at later stages (20 and 24 h), when growth was inhibited in untreated cells, increased bacterial burden was detected in L-NMMA-treated cells (Fig. 2e–g and Supplementary Fig. 2b). Similar results were also observed in macrophages isolated from iNOS−/− mice (Fig. 2h). The supplementation of NO increased intracellular burden of S. Typhimurium in iNOS−/− PMs at early infection stages (first 4–12 h) (Fig. 2i). These results indicated that host NO production promotes S. Typhimurium replication in mouse macrophages at early infection stage, while inhibiting replication at later stage. To verify whether SPI-2 is required for the action of NO on S. Typhimurium replication, the ssrB mutant, which is unable to express SPI-2 genes (Supplementary Fig. 2c), was generated and assessed for NO-dependent replication. Consistent with the essential role of SPI-2 in replication, the ssrB mutant did not replicate in untreated cells (Fig. 2j). Interestingly, the ssrB mutant did not replicate in L-NMMA-treated RAW264.7 cells at early infection stages but replicate at later stages (Fig. 2j), indicating that host NO production promotes S. Typhimurium replication at early infection stage by activating SPI-2 and inhibits replication at later stage in a manner that is independent of SPI-2 activation. Thus, NO is a host cue for SPI-2-dependent replication in mouse macrophages at early infection stages.
The contribution of host NO production to S. Typhimurium systemic infection was further investigated in vivo by comparing the bacterial burden in systemic organs (the liver and spleen) of i.p. infected C57BL/6 wild-type and iNOS−/− mice. ROS is reportedly essential for the early killing of ingested Salmonella by macrophages and RNS is involved in the control of Salmonella replication during the late stages of infection30. Consistently, both wild-type and iNOS−/− mice survived equally well for the first 6 days post-infection (Supplementary Fig. 2d). However, wild-type mice had higher bacterial burden in their liver and spleen than the iNOS−/− mice during the initial three (liver) or four days (spleen) post-infection (Fig. 2k), indicating that NO production promotes S. Typhimurium intracellular replication at the early infection stage, which was in agreement with the macrophage replication assays (in vitro results). In contrast, bacterial burden were significantly higher in iNOS−/− mice than in wild-type mice from day 5 post-infection (Fig. 2k), consistent with bacteriostatic activity of NO against Salmonella at later infection stages as reported previously30. The intravenous (i.v.) infected wild-type mice also had higher bacterial burden in their liver and spleen than iNOS−/− mice as determined at day 2 post-infection (Supplementary Fig. 2e), indicating that the NO-induced virulence promotion at the early infection stage is not associated with the infection route. These results confirmed that NO is an in vivo host signal that contributes to S. Typhimurium virulence by promoting intracellular replication at early infection stages.
S. Typhimurium SPI-2 expression levels correlate positively with bacterial replication in mouse macrophages and in vivo virulence
As host NO production demonstrated opposite effects on S. Typhimurium replication at early and late infection stages, the significance of NO contribution to S. Typhimurium replication at early infection stages was investigated. As NO promotes S. Typhimurium replication by inducing SPI-2 expression, the effect of SPI-2 expression levels on replication was assessed to determine the contribution of NO production. Seven ssrA (a master regulator of SPI-2 genes) promoter replacement derivatives were generated (Supplementary Table 2). The promoters for the replacement of ssrA promoter were selected based on their expression levels in RAW264.7 cells in the RNA-seq data. Their replacement of the ssrA promoter were performed by overlap extension PCR and Red recombination system (see Materials and methods for details). These expressed SPI-2 genes in RAW264.7 cells at various levels ranging from 5.8-fold lower to 2.6-fold higher than the wild-type level (P1150::PssrA, PsinR::PssrA, PyfiR::PssrA, PaceB::PssrA, PphnA::PssrA, P2773::PssrA, and P0658::PssrA) as verified by qRT-PCR analysis of ssrA at 8 h post-infection (hpi) (Fig. 3a). The expression of four other representative SPI-2 genes (ssaE, sscA, ssaG, and sifA) inside RAW264.7 cells correlated well with the expression of ssrA gene in each derivative strain (Supplementary Fig. 3a), indicating that the divergent expression of SPI-2 genes inside macrophages was achieved by using these derivative strains.
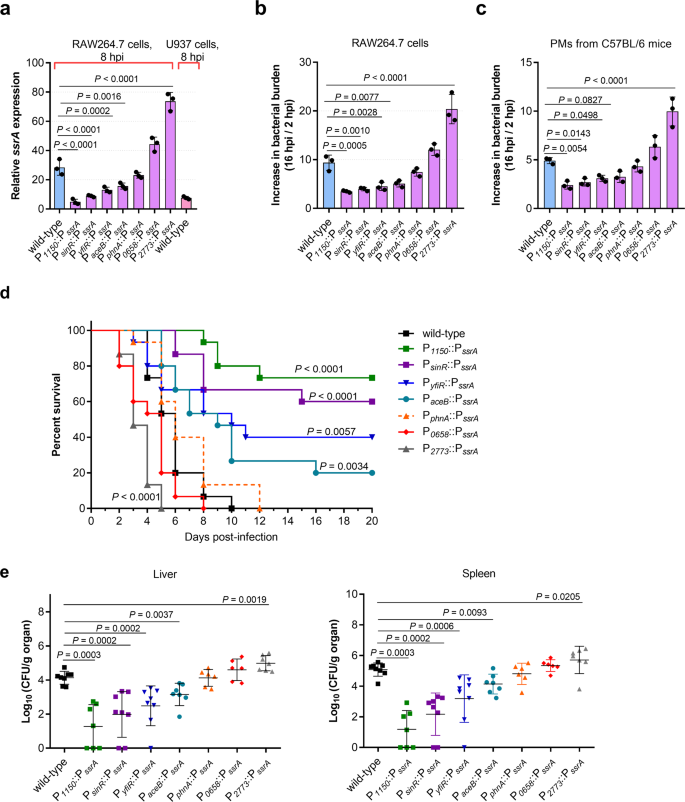
a qRT-PCR analysis of S. Typhimurium ssaG mRNA levels in different promoter–replaced strains (n = 3 independent experiments). RNA was extracted from bacteria collected from RAW264.7 cells or U937 cells at 8 hpi. RNA extracted from bacteria in the RPMI-1640 medium was used as a control. b, c Increase in bacterial burden of S. Typhimurium wild-type and promoter–replaced strains in RAW264.7 cells (b) and PMs derived from C57BL/6 mice (c) (n = 3 independent experiments). d Survival plots of mice after intraperitoneal (i.p.) inoculation with ~5×103 CFUs of wild-type or promoter–replaced strains. n = 15 mice per group. e Bacterial counts recovered from the liver and spleen of mice i.p. infected with S. Typhimurium wild-type and promoter–replaced strains. n = 6–8 mice per group. All data are presented as mean ± SD. P values were determined using two-tailed unpaired one-way ANOVA (a–c), log-rank curve comparison test (d), or Mann–Whitney U-test (e). Source data are included in Supplementary Data 1.
Macrophage replication assays were performed with the seven derivative strains in RAW264.7 cells and mouse PMs 16 hpi. At this time point, high intracellular bacterial burden and high NO production was detected (Fig. 1a and Supplementary Fig. 1c) while the death of cells was not significant (Supplementary Fig. 3b). We found that the increase in bacterial burden of wild-type S. Typhimurium and derivatives expressing similar (PphnA::PssrA) or higher SPI-2 level (P0658::PssrA and P2773::PssrA) were significantly higher than that of lower–SPI-2–expressing derivatives (P1150::PssrA, PsinR::PssrA, PyfiR::PssrA, and PaceB::PssrA) in both RAW264.7 cells and PMs, suggesting a positive correlation between SPI-2 expression levels and replication rates in macrophages (Fig. 3b, c). Lower–SPI-2–expressing derivatives (P1150::PssrA, PsinR::PssrA, PyfiR::PssrA, and PaceB::PssrA) grew as well as wild-type in LB medium (Supplementary Fig. 3c), and infected RAW264.7 cells as efficiently as wild-type (Supplementary Fig. 3d), indicating that the decreased replication ability of these derivatives in macrophages was not due to growth defect or a reduced infection ability. The results indicate that the replication level of S. Typhimurium in mouse macrophages is positively correlated with SPI-2 expression level.
Mouse infection assays were then carried out to further verify the effect of SPI-2 expression levels on S. Typhimurium virulence. The death rates of mice infected with wild-type S. Typhimurium and derivatives expressing similar (PphnA::PssrA) or higher level of SPI-2 (P0658::PssrA and P2773::PssrA) were significantly higher than that of those infected with lower–SPI-2–expressing derivatives (P1150::PssrA, PsinR::PssrA, PyfiR::PssrA, and PaceB::PssrA); all mice in the former group died by day 12 while 22–76% of mice in the latter group survived over the 20-day monitoring period (Fig. 3d). As expected, a negative correlation between SPI-2 expression levels and survival rates of infected mice was obtained (Fig. 3d). Consistently, SPI-2 expressing levels correlated positively with bacterial burden in the systemic organs (the liver and spleen) of infected mice on day 3 post-infection based on colony enumeration (Fig. 3e). The results indicate that the virulence of S. Typhimurium during systemic infection in mice is dependent on SPI-2 expression level. Thus, NO signaling is necessary for S. Typhimurium systemic infection by inducing high-level SPI-2 expression to enhance replication in mouse macrophages.
In the absence of NO signaling, SPI-2 expression was reduced 2.4-fold in RAW264.7 cells (Fig. 2a), which was similar to the SPI-2 expression level in the ssrA-replacement mutant PyfiR::PssrA (Fig. 3a). It can be inferred that the attenuated virulence due to the lack of NO signal may be similar to that of PyfiR::PssrA, which showed significantly reduced virulence in mice (Fig. 3d, e). NO production in RAW264.7 cells was not affected by the PyfiR::PssrA mutation (Supplementary Fig. 3e). Thus, lack of NO production contributes, at least partially, to the reduced virulence in humans.
Host NO promotes SPI-2 expression by activating PhoP/PhoQ in mouse macrophages
To investigate the signal transduction pathway for NO-dependent SPI-2 activation, we first considered the possible involvement of known SPI-2 regulators. As indicated by RNA-seq and confirmed by qRT-PCR, the expression of phoP and slyA (a PhoP-regulated gene) was induced to higher level in mouse than that in human macrophages (Supplementary Table 3), in accordance with the differential induction of SPI-2 genes. Inhibiting NO production by L-NMMA in mouse RAW264.7 cells and PMs resulted in reduced phoP transcription (Fig. 4a), indicating that NO production enhanced phoP transcription in mouse macrophages. Considering the essential role of PhoP in SPI-2 induction in mouse macrophages, ssaG was not expressed by the phoP mutant in both untreated and L-NMMA-treated RAW264.7 cells and PMs as expected (Fig. 4b), indicating that PhoP was required for NO-mediated SPI-2 activation. Therefore, higher SPI-2 expression in mouse macrophages was due to NO-enhanced PhoP activity. In vitro analysis further revealed that phoP/Q expression was significantly enhanced by NO (20 µM) under low O2, but not high O2, conditions (Fig. 4c, d), indicating that the response to NO under host condition (low O2) by PhoP/PhoQ was likely mediated by other regulators, such as an anaerobic regulator.
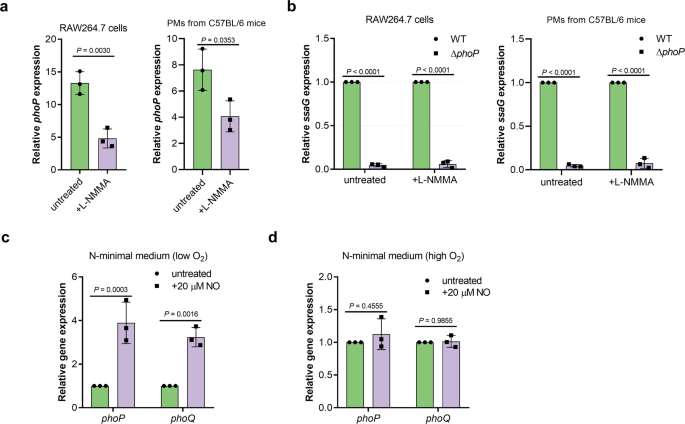
a qRT-PCR analysis of S. Typhimurium wild-type phoP mRNA levels in RAW264.7 cells or PMs in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). RAW264.7 cells or PMs were infected with wild-type strains at an MOI of 10. L-NMMA was added at a concentration of 50 µM. Fold changes in phoP gene expression in intracellular bacteria at 16 hpi relative to that in bacteria collected from the cell suspension are presented. b qRT-PCR analysis of S. Typhimurium ssaG mRNA levels in wild-type and phoP mutant in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). RAW264.7 cells or PMs were infected with wild-type or phoP mutant at an MOI of 10. L-NMMA was added at a concentration of 50 µM if indicated. At 16 hpi, fold changes in ssaG gene expression in wild-type relative to their expression in phoP mutant are presented. c, d qRT-PCR analysis of S. Typhimurium wild-type phoP and phoQ mRNA levels in the presence or absence of 20 μM NO either under low O2 (c) or high O2 conditions (d) (n = 3 independent experiments). Wild-type bacteria was grown in N-minimal medium in the presence or absence of 20 μM NO either under low O2 or high O2 conditions. Fold changes in phoP and phoQ expression in the presence of NO relative to that in the untreated samples are presented. All data are presented as mean ± SD. P values were determined using two-tailed unpaired Student's t test (a) or two-way ANOVA (b–d). Source data are included in Supplementary Data 1.
Fnr senses NO to enhance PhoP/Q-mediated SPI-2 activation in mouse macrophages
The expression of fnr, which encodes a NO-responsive global anaerobic regulator, was induced to much higher levels in mouse RAW264.7 cells (14.6-fold) than that in human U937 cells (3.7-fold) based on the RNA-seq data (Supplementary Table 4), and inhibiting NO production in mouse RAW264.7 cells using L-NMMA resulted in markedly decreased fnr transcription levels (Fig. 5a), indicating the role of NO in fnr transcription. The expression of phoP and ssaG was down-regulated by the deletion of fnr and enhanced by its overexpression in RAW264.7 cells (Fig. 5b), indicating that Fnr is required for PhoP-induced SPI-2 activation in mouse macrophages. Down-regulation and up-regulation of phoP and ssaG expression by deletion and overexpression of fnr, respectively, were also detected in vitro in bacteria cultured in N-minimal medium under low O2 condition in the absence of NO, and more strongly in the presence of 20 µM NO (Fig. 5c), indicating that Fnr-dependent PhoP/Q-mediated activation of SPI-2 is enhanced by NO production in mouse macrophages. fnr mutant showed significantly reduced bacterial burden in mouse macrophages, consistent with its role in activating phoP and SPI-2 genes (Fig. 5d).
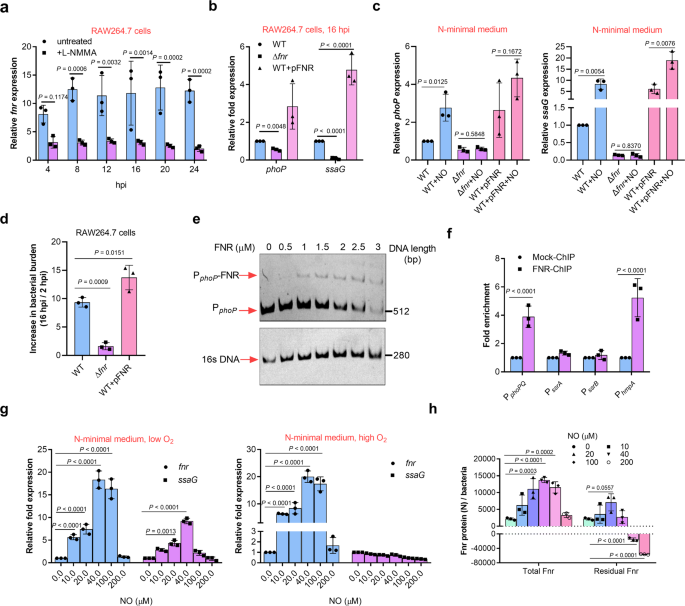
a qRT-PCR analysis of fnr mRNA levels in S. Typhimurium wild-type at indicated time points post-infection of RAW264.7 cells, in the presence or absence of 50 µM L-NMMA (n = 3 independent experiments). b qRT-PCR analysis of phoP and ssaG mRNA levels in the wild-type, fnr mutant, or fnr-overexpressing strains at 16 h post-infection of RAW264.7 cells (n = 3 independent experiments). c qRT-PCR analysis of phoP and ssaG mRNA levels in the wild-type, fnr mutant, or fnr-overexpressing strains grown in N-minimal medium in the presence or absence of 20 µM NO (n = 3 independent experiments). d Increase in bacterial burden of wild-type, fnr mutant, or fnr-overexpressing strains in RAW264.7 cells at 16 hpi (n = 3 independent experiments). e Electrophoretic mobility shift assay (EMSA) of the phoP promoter with purified Fnr protein. Images are representative of three independent experiments. Full gels of the EMSA are shown in Supplementary Fig. 4. f Fold enrichment of the phoP promoter in Fnr-chromatin immunoprecipitation (ChIP) samples (n = 3 independent experiments). g qRT-PCR analysis of fnr and ssaG mRNA levels in the wild-type strain grown in N-minimal medium in the presence of indicated NO concentrations (n = 3 independent experiments). h Concentration of the Fnr protein in the presence of indicated NO concentrations (n = 3 independent experiments). N indicate the molecular number. Wild-type S. Typhimurium were grown in N-minimal medium in the presence or absence of the NO generator spermine NONOate and under low O2 conditions. The total Fnr protein content in the bacteria was estimated using ELISA. Based on the reaction between [4Fe-4S] cluster of Fnr and eight NO molecules, the NO-inactivated Fnr molecular were calculated, and the residual functional Fnr protein molecular were calculated. All data are presented as mean ± SD. P values were determined using two-way ANOVA (a–c, f–h), or one-way ANOVA (c). Source data are included in Supplementary Data 1.
Electrophoretic mobility shift assay (EMSA) analysis showed that purified Fnr protein bound to the promoter of phoPQ in vitro (Fig. 5e and Supplementary Fig. 4). Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) analysis further demonstrated the binding of Fnr with the phoPQ promoter (Fig. 5f). These results indicate that Fnr directly activated phoPQ by binding to its promoter.
In vitro analysis further revealed that the expression of fnr increased with increase in NO concentration from 10 to 40 μM (derived from Spermine NONOate), corresponding to levels in mouse RAW264.7 cells (10–20 μM), but was not affected by higher level NO concentration (200 μM) (Fig. 5g). In addition to the NO concentration-dependent enhancement of fnr expression, ssaG expression was found to increase, but only under low O2 condition, which is in agreement with the inactivation of Fnr under high O2 conditions (Fig. 5g). Thus, Fnr senses NO production in mouse macrophages and enhances its transcription in response, leading to enhanced Fnr-mediated activation of SPI-2.
As binding to NO results in the inactivation of Fnr due to the nitrosylation of [4Fe-4S] cluster in the protein44, the NO concentration-dependent enhancement of Fnr-mediated SPI-2 activation indicated the presence of unbound Fnr proteins under low NO concentration (10–40 μM) due to increased fnr transcription. This was confirmed by estimating the total Fnr molecules produced per bacterium[calculated from the protein concentrations determined by ELISA and molar mass of Fnr (Mr 30,000) and the portion needed for maximum NO-binding (each [4Fe-4S] cluster of Fnr reacts with maximum eight NO molecules)] (Fig. 5h).
Discussion
In this study, we report that NO produced by the host innate immune system is an intracellular cue for the promotion of S. Typhimurium replication in mouse macrophages and mouse systemic organs, where it activates SPI-2 through Fnr- and PhoP/Q-mediated signal transduction pathway. The induction of NO signaling-induced SPI-2 activation in S. Typhimurium was concentration-dependent (5–20 µM), and SPI-2 expression was inhibited by high levels of NO (50–100 μM), indicating that S. Typhimurium virulence is differentially affected by the level of host NO production. The NO production in mouse macrophages was within the range required for maximum induction, while the lack of NO production possibly contributed to the absence of S. Typhimurium replication in human U937 cells, providing evidence for differential S. Typhimurium virulence in mice and humans.
In agreement with the finding in IFN-γ-activated macrophages, NO-dependent inhibition of S. Typhimurium replication at later infection stages was also detected in mouse macrophages in the present study, and further the inhibition was independent of SPI-2. Thus, host NO production acts as a double-edged sword, promoting S. Typhimurium virulence at early infection stages but inhibiting its replication at later stages. As NO is freely diffused into the SCV, it is likely that RNS derived from NO accumulate at high levels at later infection stages to inhibit S. Typhimurium replication. Supporting this hypothesis, the levels of ONOO- in RAW264.7 cells was significantly increased at 16 h post-infection (Supplementary Fig. 5), coincident to the onset of inhibition. However, further validation of this hypothesis and identification of the responsible congeners, if any, remain to be investigated.
Most of the known host environmental cues for SPI-2 activation in mouse macrophages, including mildly acidic pH, low Mg2+, antimicrobial peptides, and hyperosmotic stress reported previously50,51,52 as well as NO identified here, are mediated by PhoP/Q, further implicating PhoP/Q as an integrating point for multiple host cues for SPI-2 activation. Acidic pH, low Mg2+, antimicrobial peptides, and hyperosmotic stress are directly sensed by PhoQ, and then, the activated PhoQ promotes the PhoP active (i.e., phosphorylated) state (i.e., PhoP-P). In contrast, phoP/Q response to NO is indirect and is mediated by Fnr that directly binds to the phoPQ promoter to enhance PhoP/Q expression. In addition, the NO-mediated activation of PhoPQ occurs only under low O2 conditions, which is also a characteristic of the SCV environment. Therefore, this study identified another mechanism for the control of PhoP/Q activity.
In addition to activate via SsrA/SsrB, PhoP also activates the SPI-2 effector SseL in an SsrA/SsrB-independent manner, via directly binding to the promoter region of sseL gene53. Besides PhoP, OmpR is another important regulator of SPI-2 genes54. In response to the increase of phosphorylated OmpR, the elevated SPI-2 ssrA expression in S. Typhimurium wild-type is comparable to that of the phoP mutant, suggesting that PhoPQ might also activate SPI-2 gene expression by modulating events upstream of OmpR55. Therefore, NO-mediated PhoP activation may also regulate SPI-2 independent of SsrA/SsrB.
The finding that low NO level could enhance Fnr-mediated SPI-2 activation was unexpected, as Fnr is inactivated by binding with NO56. Our previous understanding of the impact of NO on Fnr regulation was limited to de-repressing gene expression due to inactivation of the regulator, as in the case of Hmp43,44. The present study reports that NO not only inactivates Fnr by binding to the protein but also positively regulates its transcription at a lower concentration, resulting in enhanced Fnr activity and thereby affecting Fnr-regulated genes, including PhoPQ and SPI-2. Thus, NO signaling-induced Fnr activation has a global effect on genes regulated by this protein.
The finding that NO (at levels similar to those produced by host innate immune systems)-enhanced Fnr activation in a dose-dependent manner has a huge impact on Fnr-controlled genes expression, particularly in bacterial pathogens. In the case of S. Typhimurium, activation of Fnr by NO produced by mouse macrophages, therefore, also contributes to S. Typhimurium pathogenesis by affecting other genes in a global way. The impact of low level NO on Fnr activity is far beyond SPI-2 genes and has a global impact on the Fnr regulon.
Both Fnr and PhoP/Q are ancestral global regulatory systems that affect a large number of genes and are implicated in the pathogenicity of many bacterial pathogens apart from Salmonella, such as Shigella57, uropathogenic Escherichia coli58, and avian pathogenic Escherichia coli59. The involvem...
Comments
Post a Comment