Self-collection of samples for group B streptococcus testing during pregnancy: a systematic review and meta-analysis ... - BMC Medicine
Screening results
The search identified 5909 citations (Fig. 1). A total of 3913 citations were excluded following title/abstract screening. Nine further articles were retrieved from manual searching of reference lists. On full text review, 223 citations were excluded, with reasons outlined in Fig. 1 and Additional file 4, leaving 30 articles reporting on 5 RTIs.
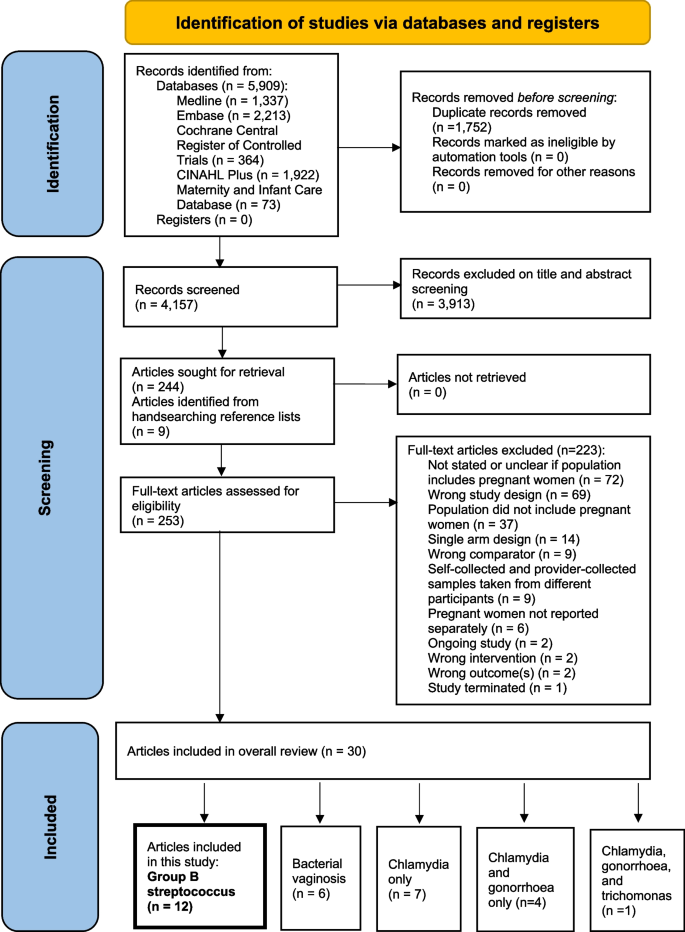
PRISMA flowchart [30] of identification and selection of studies
Characteristics of included studies
Eleven studies (3269 participants) reported in 12 articles [17, 19, 20, 31,32,33,34,35,36,37,38,39] related to GBS in pregnancy were included in the meta-analysis. Two articles [20, 38] published the results from the same study and were thus extracted collectively. Table 1 displays key study characteristics. Four were randomised cross-over trials [19, 31, 36, 39], and eight were non-randomised studies [17, 20, 32, 34, 35, 37, 38], including one non-randomised cross-over study [37]. Research integrity assessment (RIA) of the four included randomised trials [19, 31, 36, 39] are summarised in Additional file 5 [19, 31, 36, 39]—we identified "some concerns" for three trials [31, 36, 39].
No included studies which compared self-collected and provider-collected samples taken from different participants measured the outcome of maternal, perinatal, and/or neonatal outcomes, allowing comparison of risk of health outcomes between self-collection and provider-collection.
Included studies were published between 1995 and 2022 and were conducted in the US (3 studies) [20, 37,38,39], Canada (2 studies) [19, 33], Ireland (1 study) [17], Norway (1 study) [35], France (1 study) [31], Spain (1 study) [34], Hong Kong (1 study) [36], and China (1 study) [32]. All were studies of pregnant participants only, except for one study [31] in which 224/1027 participants (21.8%) were pregnant. Seven studies screened participants for GBS from 35 weeks' GA [17, 19, 32, 34,35,36, 39], one French study screened participants from 7 to 41 weeks [31], one Canadian study screened participants from 26 to 28 weeks [33], and two US studies screened participants from 24 to 42 weeks [20, 38] and at 28 weeks [37].
The delay between self-sampling and provider sampling was short, with all self-collected and provider-collected swabs taken on the same day, except for one study [32], where samples may have been taken at different visits. In the four randomised cross-over trials [19, 31, 36, 39], participants were randomised to two groups, determining the order in which self-collected and provider-collected swabs were taken, with the comparative group's swabs taken in the reverse order. One study [37] employed a similar cross-over design with participants split into two groups, but participant allocation was not randomised.
All studies tested for GBS colonisation with swab culture. The same test was performed on both self-collected and provider-collected samples. All sample collection involved either vaginal and rectal swabs [20, 36, 38], combined rectovaginal swabs [17, 19, 32, 34, 35, 37, 39], or vaginal swabs only [31]. Nine studies [17, 19, 20, 32, 33, 35,36,37,38,39] incubated specimens in enriched culture media (with or without direct plating), one study [31] used direct plating only, and one study [34] used direct plating only for 70.9% (n = 134/189) of sample pairs and enriched culture media for the remaining 29.1% (n = 55/189). The threshold for a positive test result was defined by authors as isolation of GBS on culture of either a combined vaginal-rectal swab, a vaginal swab only, or a rectal swab only.
In all studies, participants were given self-collection instructions during an in-person consultation. These instructions were verbal [17, 20, 35, 38], written or schematic [19, 31, 33, 34, 37, 39], verbal and written [32], or via video [36]. All self-collection occurred at the study clinics [17, 19, 20, 31, 32, 35,36,37,38,39], except for one study [34] in which self-collection occurred at home. In this study, participants were recruited at 30–32 weeks' GA and instructed to undertake self-collection on the same morning as their 35–37-week antenatal consultation and bring their sample to their appointment, during which provider-sampling would occur.
The overall methodological quality of the included studies was generally poor (Table 2, Additional file 6 [17, 19, 20, 31,32,33,34,35,36,37,38,39]). No studies were at low risk of bias for all domains, though several studies did not report sufficient detail for some domains. There were no concerns regarding applicability, i.e. the extent to which included studies answered the review question.
Sensitivity and specificity
Table 3 shows the pooled sensitivity (Se), specificity (Sp), diagnostic odds ratio (DOR), positive and negative likelihood ratios (LR + and LR −), and the inverse of the negative likelihood ratio (1/LR −) for the eleven included studies [17, 19, 20, 31,32,33,34,35,36,37,38,39]. Figure 2 displays the HSROC curves of the eleven studies. Figure 3 displays forest plots of the accuracy of GBS self-collection compared to provider-collection. We analysed randomised trials (4 studies, 1226 participants) and non-randomised studies (7 studies, 2043 participants) separately. Among the four randomised trials, pooled sensitivity was 82% (95% CI: 66–91%) with point estimates ranging from 60 to 94%, and pooled specificity was 99% (95% CI: 98–99%) with point estimates of 99%. In the seven non-randomised studies, pooled sensitivity was 91% (95% CI: 83–96%) with point estimates ranging from 75 to 100%, and pooled specificity was 97% (95% CI: 94–99%) with point estimates ranging from 86 to 100%.
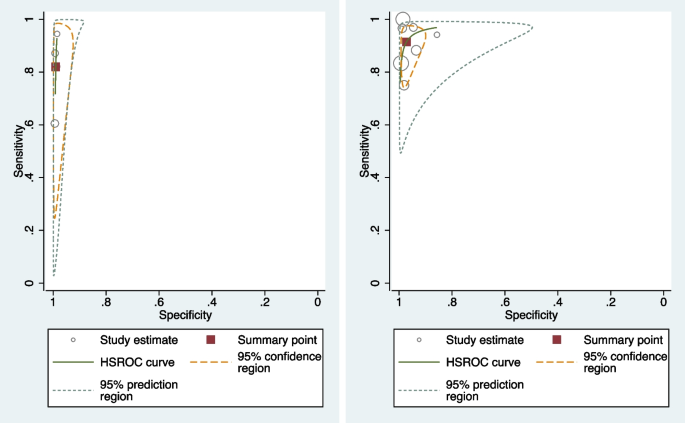
HSROC plots of randomised (left) and non-randomised (right) GBS studies that compared self-collection to provider-collection: The circles represent individual study estimates, with circle size proportional to sample size of each study. The continuous green line is the summary curve from the model. The red square represents the summary estimate for sensitivity and specificity and the yellow dotted line represents the 95% confidence region for this summary estimate. The green dotted line represents the 95% prediction region
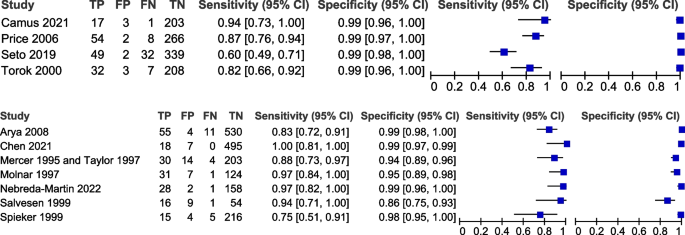
Forest plots of randomised (top) and non-randomised (bottom) studies that compare self-collected samples to provider-collected samples for GBS testing
On visual inspection of forest plots (Fig. 3) to compare studies that could have been part of subgroups, no obvious trends were observed.
In the randomised trials, heterogeneity for sensitivity (68.85%) was higher than that of specificity (12.08%), and for non-randomised studies, heterogeneity for specificity (72.50%) was higher than that of sensitivity (37.38%) (Table 4). Despite the high I2 for sensitivity and specificity, the generalised between-study I2 was close to zero, which can be attributed to the correlation between sensitivity and specificity (rho = − 1.00 and − 0.51) [40].
Sensitivity analyses are detailed in Additional file 7 [20, 33, 34, 37, 38].
Positive test prevalence is displayed in Table 1. A summary of all other additional outcomes is detailed in Additional file 8 [17, 19, 20, 31,32,33,34,35,36,37,38,39].
Comments
Post a Comment