The causal effect of Helicobacter pylori infection on coronary heart disease is mediated by the body mass index: a ... - Nature.com
Abstract
The association between Helicobacter pylori (H. pylori) infection and coronary heart disease (CHD) remains controversial, with an unclear causal link. This study employed bidirectional Mendelian randomization (MR) method, using H. pylori infection as the exposure, to investigate its causal relationship with CHD diagnosis, prognosis, and potential pathogenesis. H. pylori infection exhibited a causal association with body mass index (BMI) (β = 0.022; 95% CI 0.008–0.036; p = 0.001). Conversely, there was no discernible connection between H. pylori infection and the diagnosis of CHD (OR = 0.991; 95% CI 0.904–1.078; p = 0.842; IEU database; OR = 1.049; 95% CI 0.980–1.118; p = 0.178; FinnGen database) or CHD prognosis (OR = 0.999; 95% CI 0.997–1.001; p = 0.391; IEU database; OR = 1.022; 95% CI 0.922–1.123; p = 0.663; FinnGen database). Reverse MR analysis showed no causal effect of CHD on H. pylori infection. Our findings further support that H. pylori infection exerts a causal effect on CHD incidence, mediated by BMI. Consequently, eradicating or preventing H. pylori infection may provide an indirect clinical benefit for patients with CHD.
Similar content being viewed by others
Cardiovascular effects of the post-COVID-19 condition

Association of semaglutide with risk of suicidal ideation in a real-world cohort
Cardiovascular autonomic dysfunction in post-COVID-19 syndrome: a major health-care burden
Introduction
Coronary heart disease (CHD) is caused by atherosclerosis, which includes angina pectoris and myocardial infarction (MI) and is the leading cause of mortality in many countries1. The etiology, pathogenesis and prognosis of CHD are complex and have not been fully understood until recently. Helicobacter pylori (H. pylori) is a gram-negative bacterium that primarily inhabits the stomach and duodenum2. More than half of the world's population has been infected with H. pylori3. In addition to causing gastrointestinal diseases4, H. pylori can also induce systemic reactions, including abnormal glucose5 and lipid metabolism6, heightened blood hypercoagulability7,8, and chronic inflammatory reactions9,10,11, and is accompanied by vitamin (including vitamin B12, vitamin C, and vitamin D) deficiency12. While these reactions represent risk factors for CHD, it remains uncertain whether H. pylori influences the occurrence of CHD through these reactions.
However, the relationship between H. pylori infection and CHD is still controversial. Several studies have shown that H. pylori infection is not significantly related to the occurrence or severity of CHD13,14; however, some studies have shown that H. pylori infection is one of the main causes of CHD15,16. Studies have reported that eradication therapy for H. pylori can reduce the levels of peripheral blood inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) in patients. These inflammatory cytokines are implicated in the development of atherosclerosis and CHD, and their elevation increases the incidence of restenosis in patients after percutaneous transluminal coronary angioplasty (PTCA)17,18,19. The probability of MI in H. pylori-infected patients is twice that in uninfected individuals20. Another study used infrared radiation spectroscopy to measure the levels of triglycerides, C-reactive protein, homocysteine, low-density lipoprotein (LDL), and TNF-α in peripheral blood. The results showed that, compared with healthy individuals, CHD patients with H. pylori infection had elevated triglyceride levels and inflammation21. An Asian study also confirmed that H. pylori infection can increase the risk of CHD in the next 10 years22. At present, the evidence for a link between H. pylori infection and CHD is based on observational studies, and there may be some unknown confounding factors that affect judgment of the results. To address this controversial clinical issue, a study that removes confounding factors to accurately determine the causal relationship between H. pylori infection and CHD is urgently needed. In addition, although the infection rate of H. pylori is relatively high, H. pylori infection is not routinely screened, and many infected individuals are unaware of having this infection. Exploring the causal relationship between the two will help determine whether routine screening and treatment of H. pylori is one of the prevention and treatment strategies for CHD.
Mendelian randomization (MR) has emerged as a popular epidemiological statistical method that can remove confounding factors and accurately determine the causal relationship between two variables. The method relies on the use of the public genome-wide association study (GWAS) database to obtain instrumental variables (IVs) that are strongly related to exposure but are not related to outcomes or confounding factors. IVs are usually single nucleotide polymorphisms (SNPs), and the causal relationship between exposure and outcomes can be accurately inferred using IVs. In this study, we used H. pylori infection as the exposure and applied the bidirectional MR method to infer the relationship between H. pylori infection and the diagnosis, prognosis, and possible pathogenesis of CHD. We also used CHD as the exposure to explore the reverse causal relationship between CHD and H. pylori infection, two step MR analyses were used to explore indirect pathogenic factors of H. pylori infection, with the aim of clarifying this relationship and providing clinical suggestions for the diagnosis and treatment of CHD, providing new insights for CHD.
Methods
Study design
For the current study, we used IVs as a proxy for exposure, and then conducted an MR analysis to test the association between exposure and outcome23. MR is based on three principle assumptions: (1) correlation assumption: IVs are strongly correlated with exposure; (2) exclusivity hypothesis: IVs are not associated with outcomes; and (3) independence hypothesis: IVs are independent of other confounding factors24 (Fig. 1).
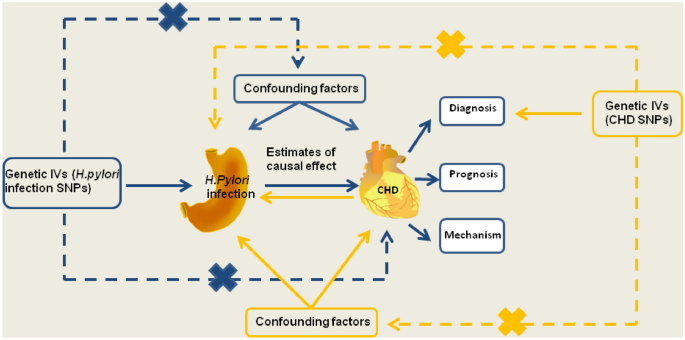
Schematic representation of the MR study on the causal relationship between H. pylori infection and CHD incidence. CHD, coronary heart disease; IVs, instrumental variables; H. pylori, Helicobacter pylori; SNP, single-nucleotide polymorphism.
Description of the data sources
The genetic association of CHD was derived from the CARDIoGRAMplusC4D Consortium, which included 60,801 cases and 123,504 control subjects from 48 studies, and of which 77% of the participants were of European ancestry and 19% were of South and East Asian ancestry25. We also collected summary statistics for CHD, MI and angina pectoris, which were derived from the FinnGen database (https://www.fifinngen.fifi/en)26. H. pylori infection data were derived from the European Bioinformatics Institute (EBI) database (https://gwas.mrcieu.ac.uk/datasets/ieu-b-4905/) and included 1058 cases and 3625 controls. GWAS data were also collected to investigate the causal effect between H. pylori infection and the prognostic data for CHD, including major adverse cardiovascular events (MACE; Neale laboratory and FinnGen database), heart failure (Neale laboratory), heart arrhythmia, heart attack, stroke, target heart rate (HR) reached, and maximum HR data [MRC Integrated Epidemiology Unit (MRC-IEC), https://www.bristol.ac.uk/integrative-epidemiology]. In addition, GWAS data on the possible pathogenesis between H. pylori and CHD were also obtained, including fasting blood glucose data from the EBI database, body mass index (BMI) data from the MRC-IEU database, and lipid trait data from the UK Biobank database. Vitamin data were obtained from the MRC-IEU database. Inflammation data were downloaded from the public database IEU (https://gwas.mrcieu.ac.uk/). The GWAS data are detailed in Table 1 and have been approved by the author or the Consortium.
The demographic characteristics of GWAS data for H. pylori infection are as follows: pregnant women residing in Avon, UK, with expected delivery dates between April 1, 1991, and December 31, 1992, were invited to participate in the ALSPAC study. The overall sample size for analyses, incorporating data collected after the age of seven, was determined. Serum antibody levels related to H. pylori infection were measured using ELISA, ultimately providing GWAS data associated with H. pylori27. In the FinnGen database, the average age of GWAS data is 63 years, with a male proportion of 43.5% (source: https://www.nature.com/articles/s41586-022-05473-8). For the UKB database, the average age of GWAS data is 56.9 years, with a male proportion of 45.8%28. The remaining GWAS datasets may have been obtained through meta-analysis, making it challenging to acquire information on gender and age.
Selection of genetic IVs for H. pylori Infection
The genetic IVs were acquired from previous literature29,30,31. This study involved bidirectional MR analysis of H. pylori infection and non-alcoholic fatty liver disease. The SNPs rs368433 and rs10004195, located in the Toll-like receptor 10 (TLR10) gene (4p14) and the Fc gamma RIIA (FCGR2A) gene (1q23.3), respectively, have been reported to be strongly associated with H. pylori infection and are used as IVs29. Instrument strength was evaluated using the F-statistic for each allele, and if the F-statistic was greater than 10, it was considered that the potential weak instrument bias was minimized30,31. The F-statistic for each SNP was derived from the following equation:
where R2 is the proportion of variation explained by IVs, N is the sample size of the exposure dataset, and MAF indicates the minor allele frequency. In our study, all F-statistics were greater than 100 and, therefore, suitable for our analysis (Supplementary Table S1).
Selection of genetic IVs for CHD and BMI
The genetic IVs for CHD and the potential pathogenesis of H. pylori infection were obtained from the GWAS summary statistics. The following three steps were subsequently used to screen for strong correlations with CHD but not with H. pylori infection or confounding factors to ensure that the effect of each allele (containing each SNP) was the same. First, SNPs strongly related to exposure were screened (p < 5 × 10˗8). Second, independence was set to remove linkage disequilibrium (LD; r2 < 0.001, window size = 10,000 kb, p < 5 × 10˗8) and calculate the statistical strength (F-statistical > 10). Third, the exposure and outcome datasets were harmonized to ensure that the effect alleles belonged to the same allele. The SNPs screened by these strict procedures can be used as IVs for subsequent analysis (Supplementary Table S2). The genetic IVs for BMI were obtained by the same screening method (Supplementary Table S3).
Statistical analysis and data visualization
All analyses were performed using R programming software (R4.1.2, https://www.rproject.org/). The primary MR analysis was conducted using the Wald ratio and the inverse variance weighting (IVW) method, and a two-sided p-value < 0.05 was considered indicative of statistical significance. Due to the multiple comparisons, we further applied a Bonferroni corrected threshold for statistical significance (0.05/number of analyses)32 (Table 2). In reverse MR analysis and two step MR analysis, because of the large number of IVs, we applied two complementary methods (MR‒Egger and weighted-median) to increase the stability of the results. MR analyses were performed using the R-based package "TwoSampleMR" (version 0.5.6). Forest plots were generated using the "ggplot2" R package (version 3.4.0).
Results
Causal effect of H. pylori infection on the diagnosis of CHD
According to previous studies, the SNPs rs10004195 (T>A) and rs368433 (T>C) are strongly related to H. pylori infection30. Conventional IVs typically consist of two or more. Although there were only two IVs in this study, these two SNP loci were strongly correlated with H. pylori infection, with F values greater than 100, and their efficacy was more than 10 times that of conventional IVs (Supplementary Table S1). The two corresponding genes are TLR10 and FCGR2A. TLR10 is a key gene that regulates the release of inflammatory factors during H. pylori infection33, and FCGR2A is also a key gene that regulates the intestinal34 and cardiac inflammatory responses35. We therefore used these two SNPs as IVs of H. pylori infection to predict the relationship between H. pylori infection and the diagnosis of CHD, MI, or angina pectoris29. H. pylori infection was not associated with the occurrence of CHD (IEU) [odds ratio (OR), 0.991; 95% confidence interval (CI) 0.904–1.078; p-value = 0.842], CHD (Finn) (OR, 1.049; 95% CI 0.980–1.118; p-value = 0.178), angina pectoris (OR, 1.105; 95% CI 1.019–1.191; p-value = 0.023), or MI (OR, 0.993; 95% CI 0.896–1.091; p-value = 0.889) according to the IVW method. (Fig. 2, Supplementary Table S4). Although the causal analysis between H. pylori infection and angina pectoris showed a p value < 0.05, it is imperative to consider that this study included four distinct outcomes, each subjected to separate analyses. In accordance with the Bonferroni threshold correction method, the adjusted significance level dictates that the effective p value should be < 0.0125 to account for the multiple comparisons conducted (Table 2). Therefore, our analysis revealed that there is no causal relationship between H. pylori infection and CHD diagnosis.
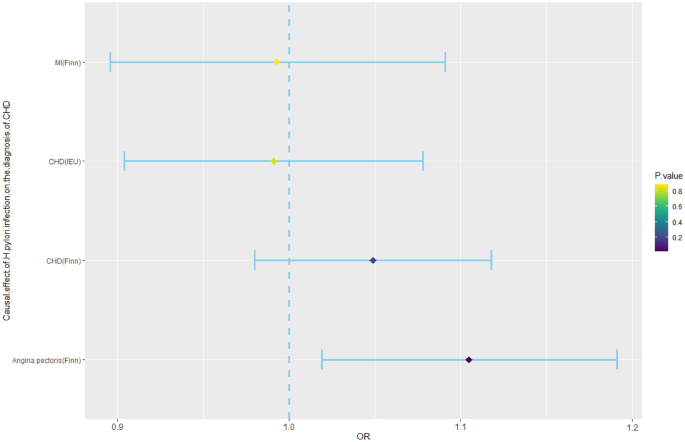
Mendelian randomization results for the effect of H. pylori infection on the diagnosis of CHD. CHD, coronary heart disease; H. pylori, Helicobacter pylori; MI, myocardial infarction; OR, odds ratio.
Causal effect of H. pylori infection on the prognosis of CHD
MR analyses were further performed to examine the causal association between H. pylori infection and the prognosis of CHD, including MACE, heart arrhythmia, heart attack, stroke, heart failure, target HR achieved, and maximum HR during the fitness test. The incidence of MACE, which mainly includes heart arrhythmia, heart attack, stroke, and heart failure, is currently the main method for determining the prognosis of CVD patients. In addition, the maximum HR and target HR in cardiopulmonary exercise tests can also predict the prognosis of CHD patients and are negatively correlated with their prognosis36,37. Therefore, this study used the above factors as prognostic indicators for CHD. The analysis showed that H. pylori infection had no causal effect on MACE (OR, 0.999; 95% CI 0.997–1.001; p-value = 0.391; IEU database; OR, 1.022; 95% CI 0.922–1.123; p-value = 0.663; FinnGen database), heart arrhythmia (OR, 1.000; 95% CI 0.999–1.001; p-value = 0.823), heart attack (OR, 0.998; 95% CI 0.996–1.000; p-value = 0.124), stroke (OR, 0.999; 95% CI 0.998–1.001; p-value = 0.525), heart failure (OR, 1.000; 95% CI 0.999–1.001; p-value = 0.741), target HR achieved (OR, 0.994; 95% CI 0.983–1.005; p-value = 0.252), or maximum HR (OR, 0.972; 95% CI 0.937–1.007; p-value = 0.115) (Fig. 3, Supplementary Table S5).
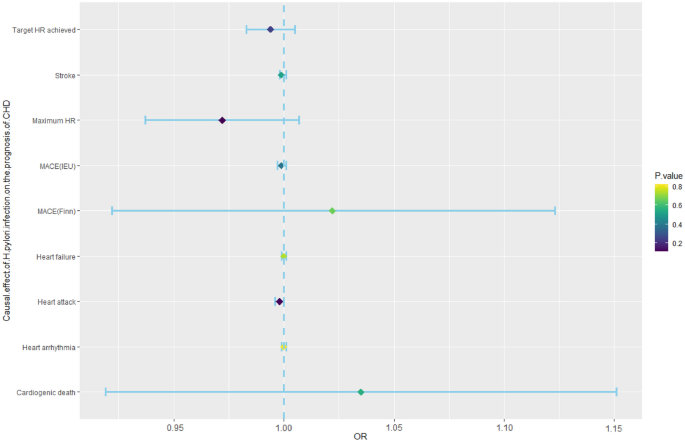
Mendelian randomization results for the effect of H. pylori infection on the prognosis of CHD patients. CHD, coronary heart disease; H. pylori, Helicobacter pylori; MACEs, major adverse cardiovascular events; Maximum HR, maximum heart rate during fitness test; OR, odds ratio; Target HR achieved, reached target heart rate.
Causal effect of H. pylori infection on the pathogenesis of CHD
Based on previous research, we summarized the pathogenic mechanisms of H. pylori infection on CHD, and these included abnormal glucose and lipid metabolism, vitamin deficiency (including vitamin B12, vitamin C, and vitamin D), and chronic inflammatory reactions. In addition to metabolic abnormalities and chronic inflammation, vitamin deficiency has also been found to be associated with the occurrence and development of CHD. H. pylori infection can cause damage to gastric wall cells, leading to a decrease in the secretion of endogenous factors by gastric wall cells and a decrease in the absorption of vitamin B12 in the small intestine. Moreover, deficiencies in vitamin C and vitamin D, both of which are associated with the H. pylori infection progression, represent risk factors for CHD. Therefore, we used these factors as indicators of CHD pathogenesis38,39. To explore the causal relationship between H. pylori infection and CHD pathogenesis, we used H. pylori infection as the exposure and pathogenesis as the outcome for MR analysis. According to the MR analyses of abnormal glucose and lipid metabolism, H. pylori infection had no association with fasting blood glucose levels (β, 0.006; 95% CI − 0.011 to 0.023; p-value = 0.511), triglyceride (TG) levels (β, 0.005; 95% CI − 0.006 to 0.016; p-value = 0.409), high-density lipoprotein cholesterol (HDL-C) levels (β, − 0.006; 95% CI − 0.047 to 0.035; p-value = 0.788), or low-density lipoprotein cholesterol (LDL-C) levels (β, 0.013; 95% CI − 0.026 to 0.051; p-value = 0.515). In the vitamin deficiency MR analysis, we obtained negative results for water-soluble vitamins, including vitamin C (β, − 0.002; 95% CI − 0.006 to 0.002; p-value = 0.318) and vitamin B12 (β, 0.008; 95% CI − 0.029 to 0.044; p-value = 0.685). The same result was also observed for the fat-soluble vitamins of vitamin D (β, − 0.0003; 95% CI − 0.003 to 0.002; p-value = 0.775). In addition, we also analyzed whether H. pylori infection contributed to the occurrence of CHD through inflammatory mechanisms and found no causal relationships between H. pylori infection and IL-4 (β, − 0.066; 95% CI − 0.258 to 0.125; p-value = 0.497), IL-6 (β, − 0.041; 95% CI − 0.117 to 0.035; p-value = 0.294), IL-8 (β, 0.017; 95% CI − 0.055 to 0.088; p-value = 0.645), IL-10 (β, − 0.079; 95% CI − 0.276 to 0.117; p-value = 0.429), IL-18 (β, 0.022; 95% CI − 0.041 to 0.086; p-value = 0.493) or TNF-α (β, 0.020; 95% CI − 0.275 to 0.316; p-value = 0.893). However, there was a significant causal relationship between H. pylori infection and BMI (β, 0.022; 95% CI 0.008–0.036; p-value = 0.001), and there was a causal relationship between BMI and CHD incidence (Fig. 4, Supplementary Tables S6, and S7). A study showed that, compared to those in the control group, patients infected with H. pylori had increased growth hormone levels and decreased obesity, which promoted appetite increase40. Another study suggested that H. pylori can affect appetite and dietary habits through the brain-gut axis41. Therefore, we speculate that the mechanism by which H. pylori promotes an increase in BMI is through the brain-gut axis to alter appetite and promote energy intake.
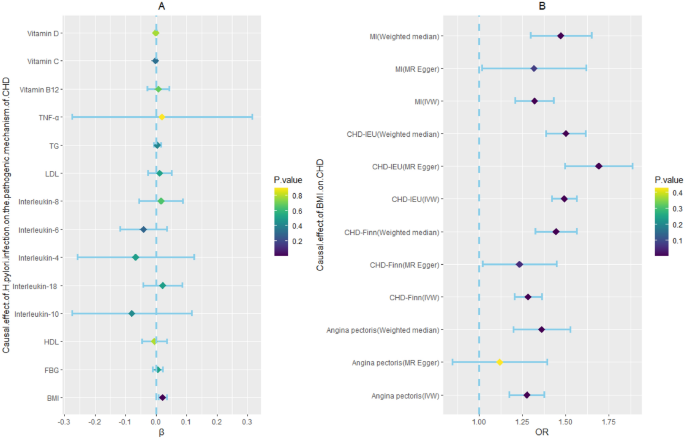
Two step Mendelian randomization results for the effect of H. pylori infection on CHD incidence (the pathogenic mechanism of CHD). BMI: body mass index; CRP, C-reactive protein; FBG, fasting blood glucose; H. pylori, Helicobacter pylori; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio. TG, triglyceride; TNF-α, tumor necrosis factor-α. Dark blue dots represent significant differences as indicated by the P values.
Reverse causal effect of CHD on H. pylori infection
The IVs of CHD, MI, and angina pectoris were identified from public GWAS summary data. Three MR analysis methods, namely, IVW, weighted median, and MR‒Egger, were used for this analysis. None of the three methods had a significant causal effect on H. pylori infection (Fig. 5, Supplementary Table S8).
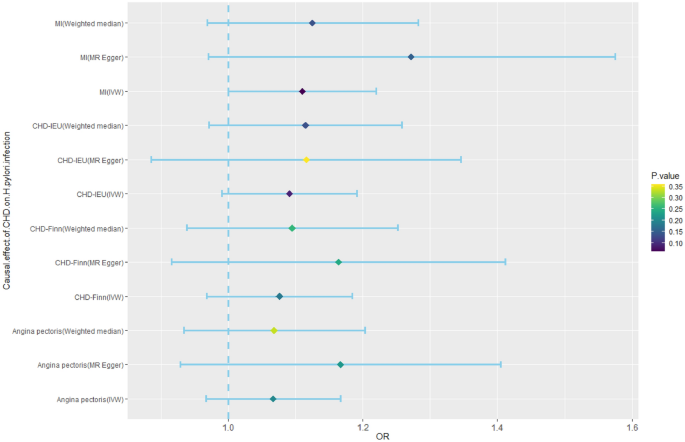
Mendelian randomization results for the effect of CHD on H. pylori infection. CHD, coronary heart disease; H. pylori, Helicobacter pylori; MI, myocardial infarction; OR, odds ratio.
Discussion
In this study, we used large-scale public GWAS data to analyze the causal relationship between H. pylori infection and the risk of CHD using the MR method. The causal effect of H. pylori infection on CHD incidence was mediated by BMI.
The association between H. pylori infection and CHD is currently controversial. Several studies have reported that H. pylori infection is related to the occurrence and prognosis of CHD19,42,43. A prospective study revealed that H. pylori-infected patients had an increased occurrence of CHD44 and adverse events42. According to other studies, MI patients infected with H. pylori have a higher mortality rate45, and the probability of restenosis after PTCA is higher17. It has also been shown that MI has a reverse causal effect on H. pylori. Young people with MI have twice the probability of H. pylori infection as healthy individuals46,47. However, some studies have been unable to detect a correlation between the occurrence and development of CHD and H. pylori infection, especially among older individuals48. A prospective study with a small sample14 and meta-analyses of five large samples14,49 have provided evidence that H. pylori infection is not significantly related to the severity or prognosis of CHD. A prospective study involving 180 patients who underwent stent implantation in a native coronary artery revealed that there is no significant association between H. pylori infection and restenosis following PTCA50. However, the pathogenic link between H. pylori infection and CHD remains controversial. First, in terms of metabolism, the influence of H. pylori infection on glucose and lipid metabolism and BMI is controversial. Regarding lipids, a study showed that H. pylori infection can reduce the level of HDL and increase the levels of LDL and TG51. However, other studies have presented opposite findings52,53. Meta-analyses and prospective studies of large samples have shown that eradication of H. pylori infection has no significant effect on the levels of HDL, TG, or LDL6,54. In terms of glucose metabolism, evidence suggests that H. pylori infection may participate in the onset of diabetes and impaired glucose control in diabetes patients55,56. Infection with H. pylori can increase insulin resistance in both young people and diabetes patients57. One study revealed that, compared with that in the control group, the improvement in glucose homeostasis in diabetes patients after successful eradication of H. pylori infection was not statistically significant58. In terms of body weight, the eradication of H. pylori infection has been associated with increased weight in children59 and has variable effects on weight in adults—either increasing60 or decreasing61 it. Additionally, there is a higher observed incidence of H. pylori infection among obese individuals62. Second, H. pylori infection induces alterations in the gastrointestinal microenvironment, potentially impeding the absorption of nutrients, resulting in a deficiency of micronutrients63. Poor vitamin B12 absorption has been shown to be related to H. pylori infection64. The levels of vitamin C and vitamin D are closely related to CHD incidence65, but the relationship between vitamins and H. pylori infection remains to be confirmed. Third, H. pylori can cause an inflammatory reaction. Chronic inflammation caused by H. pylori may have dual effects. On the one hand, low-grade inflammation is a common feature of obesity, diabetes, insulin resistance, and dyslipidemia, and H. pylori may cause a chronic inflammatory reaction through abnormal metabolism66. On the other hand, H. pylori causes damage to the gastrointestinal tract67, stimulating an increase in interleukin levels10,11. H. pylori infection has been associated with elevated levels of TNF-α and IL-6 in patients with CHD68,69,70,71. Conflicting data have also been reported regarding inflammation72. The factors involved in the pathogenesis of H. pylori infection, which include glucose and lipid metabolism, vitamin deficiency, and chronic inflammatory reactions, are all causes of CHD.
The discrepancy between H. pylori infection and CHD could be attributed to multiple factors, such as differences in the race and age of the selected sample population, the small sample size, the low incidence of MACE, the detection method for H. pylori infection, and the different follow-up times. These confounding factors may lead to the poor statistical efficiency of the data and may affect the reliability of the experimental results.
This study revealed that H. pylori infection has no direct causal effect on the diagnosis or prognosis of CHD. According to our analysis of pathogenesis, H. pylori infection has a causal effect on BMI, and BMI has a causal effect on CHD incidence. Therefore, the causal effect of H. pylori infection on CHD incidence is mediated by BMI. However, H. pylori infection has no causal effect on inflammatory factors (IL-4, IL-6, IL-8, IL-10, IL-18, or TNF-α), vitamins (vitamin B12, vitamin C, or vitamin D), or glucose and lipid metabolism, and there is no reverse causal effect of CHD on H. pylori infection.
This study used the MR method to reveal a bidirectional causal relationship between H. pylori infection and CHD for the first time and could increase the recognition of pathogenic factors of CHD from the perspective of systems biology. The advantage of MR studies is that the sample size is large, and they involve a natural randomized controlled trial, which eliminates confounding factors. However, this study has several limitations. The GWAS of H. pylori infection was based on serological samples, which may not be truly representative of H. pylori infection. Furthermore, the samples were obtained from individuals of European ancestry and therefore may not be representative of all populations worldwide. Finally, the screening of IVs in this study was strict, which may have led to negative results.
Conclusions
Our findings confirm that the causal effect of H. pylori infection on CHD incidence is mediated by BMI. Therefore, the eradication or prevention of H. pylori infection may indirectly benefit patients with CHD indirectly in the clinic.
Similar content being viewed by others

Influence of Helicobacter pylori infection on risk of rheumatoid arthritis: a nationwide population-based study
Prevalence of H. pylori among patients undergoing coronary angiography (The HP-DAPT prevalence study)
Investigating the effects of statins on ischemic heart disease allowing for effects on body mass index: a Mendelian randomization study
Data availability
All data generated or analysed during the study is included in this published article. The datasets for this study are shown in Table 1.
References
Nichols, M., Townsend, N., Scarborough, P. & Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 35, 2950–2959. https://doi.org/10.1093/eurheartj/ehu299 (2014).
Comments
Post a Comment