Multidrug-Resistant Bacterial Colonization and Infections in Large ... - CDC
Bacterial superinfections represent a major threat for patients in intensive care units (ICUs), severely affecting clinical course and length of hospital stay. The COVID-19 pandemic caused an unprecedented rate of ICU admissions and drastically changed ICU care itself, in terms of infection control measures and therapeutic usage of steroids and immunomodulating drugs. The percentages of hospital-acquired infections (HAIs) in COVID-19 patients vary widely, ranging from 7% to 13% in nonintensive hospital wards and up to 45% in ICUs (1–3).
Several studies have assessed the burden of multidrug-resistant organisms (MDROs) in COVID-19 patients admitted to ICUs, reporting heterogeneous results with prevalence ranging from 11% to 50% and incidence rate from 4.5 cases/1,000 patient-days to 30 cases/1,000 patient-days (4–21). However, studies published so far have relevant limitations, often not clearly discriminating between colonization and infection (8,9,11,12), and either including small populations or showing heterogeneity in clinical settings and microbiologic surveillance procedures when describing larger pool of persons, such as in multicentric studies (18–20).
Our study was conducted to address the need for further evidence on incidence and etiology of MDRO colonization and infections in mechanically ventilated COVID-19 patients. We analyzed clinical and microbiologic data systematically collected in a large ICU in northern Italy.
Methods
Study Design and Setting
We conducted a retrospective cohort study on routinely collected data of COVID-19 patients admitted to the Milano Fiera ICU during October 23, 2020‒May 31, 2021. This ICU is a large COVID-19 ICU developed in Milan, Italy, to face the effect of the pandemic. It admitted patients who had SARS-CoV-2 infection requiring mechanical ventilation from different healthcare settings: emergency department, nonintensive hospital wards, and other ICUs. This ICU could accommodate up to 100 patients divided into distinct units (modules) managed by ICU staff from different hospitals. Microbiologic surveillance was standardized and consisted of perineal and nasal swab specimens for MDROs and endotracheal aspirate cultures obtained at ICU admission and then once (perineal and nasal swab specimens) or twice (endotracheal aspirate) a week. All modules referred to the IRCCS Ca' Granda Ospedale Maggiore Policlinico Foundation for laboratory and microbiologic analyses and for infectious diseases specialist consultation.
Study Participants and Data Collection
All consecutive patients who had laboratory-confirmed SARS-CoV-2 infection and were admitted to the ICU were considered for inclusion. Exclusion criteria were age <18 years, length of mechanical ventilation <48 h, and lack of comprehensive clinical documentation. We collected demographic, clinical, laboratory, and outcome data from clinical records and microbiologic and therapeutic data from dedicated hospital databases (Appendix). The study was registered by the Milan Area 2 Ethical Committee (#701_2021) and was conducted in accordance with standards of the Helsinki Declaration. Written informed consent was waived because of the retrospective nature of the analysis. The study was retrospectively registered at clinicaltrials.gov on March 24, 2022 (identifier: NCT05293418).
Microbiologic Data Processing
For each patient, we retrieved bacterial isolates from a microbiology database, which were independently reviewed by dedicated intensivists and infectious disease specialists and classified as contamination, colonization, or infection, according to international guidelines (Appendix) (22,23). In brief, infections were defined by the presence of a major bacterial load associated with clinical manifestations within the infection window period (±3 days from specimen collection) (22,23), Isolates were classified as colonization when no adverse clinical signs or symptoms were documented. We defined contamination as all microbiologic isolates that did not meet the criteria of infection or colonization and that were listed in the US Centers for Disease Control and Prevention National Healthcare Safety Network (https://www.cdc.gov/nhsn/index.html) list of common commensals. We retained only the first species-specific MDRO colonization of each patient for further analysis.
We distinguished new infectious episodes from persistent infections according to the European Centre for Disease Prevention and Control definitions (23). We stratified infection episodes as infection without sepsis, sepsis or septic shock according to Sepsis-3 criteria (24). We defined secondary bloodstream infections (BSIs) by using the secondary BSI attribution period according to the Centers for Diseases Control and Prevention National Healthcare Safety Network (22). We also defined isolates as MDROs when they were nonsusceptible to >1 agents in >3 antimicrobial drug categories (25) or when harboring specific antimicrobial drug resistance mechanisms (e.g., methicillin-resistant Staphylococcus spp., vancomycin-resistant Enterococcus spp., extended-spectrum β-lactamase/AmpC/carbapenemases‒producing Enterobacterales) by using rapid detection methods (4).
Statistical Analysis
We reported patient characteristics overall and for selected groups of interest, such as MDROs acquired before/after ICU admittance and MDRO infection/colonization. Medians (interquartile range [IQRs]) are reported for continuous variables and numbers (percentages) for categorical variables. We calculated crude incidence rates (IRs) per 1,000 patient-days and relative 95% CIs, considering for each patient any first species-specific MDRO colonization or each new MDRO/non-MDRO HAI (26). We used SAS version 9.4 software (SAS Institute, https://www.sas.com) for statistical analysis (Appendix).
Results
Population Description
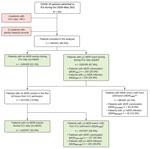
A total of 451 patients from 46 different hospitals were admitted to ICUs during October 2020‒May 2021. Of those, 435 were included in the analysis. We provide details of the patient selection process (Figure 1) and trends of patient admission by referring hospital per month (Appendix Figure 1).
Only 12/435 patients (2.7%) were reported to have MDRO colonization/infection before ICU admission. In 88/435 patients (20.2%), MDRO were isolated within 48 h upon entry to the ICU (MDR≤48h), and those patients were similarly distributed between referring hospitals (Appendix Figure 2). This group was composed of 78 colonizations and 10 infections; 35/78 (44.9%) colonized patients subsequently had MDRO infections develop. Compared with the 347 patients who had no evidence of MDRO during the first 48 hours of ICU stay (no-MDR+MDR>48h), the MDR≤48h group was characterized by higher admittance from other ICUs and lower admittances from emergency departments (ICU 31/88 [35.2%] in MDR≤48h vs. 86/347 [24.8%]) in no-MDR+MDR>48h; emergency department 15/88 [17.1%] in MDR≤48h vs. 102/347 [29.4%] in no-MDR+MDR>48h). The MDR≤48h group showed slightly longer (although not significantly) length of stay in the ICU of origin than patients who developed MDRO events later during their stay and to no-MDR patients (medians 11.5, 9, and 7 days, respectively; p = 0.09). The MDR≤48h group was also characterized by a larger amount of antimicrobial drug intake before ICU admission (no antimicrobial drug in 25/88 [28%] of MDR≤48h vs. 126/327 [36.3%] of no-MDR+MDR>48h; >3 classes of antimicrobial drugs in 12/88 [13.6%] of MDR≤48h vs. 23/347 [6.6%] of no-MDR+MDR>48h). We compiled demographic and clinical characteristics by groups (Appendix Table 1) and duration between hospitalization and transfer to the ICU on the basis of patients' setting of provenance (Appendix Table 2).
Of the 347 patients who had no MDRO isolates within the first 48 hours from ICU admission, 207 (67.5%) had >1 MDRO event (MDR>48h); 107 (30.8%) patients had MDRO colonization only (MDRCOL>48h) and 100 (28.8%) had >1 MDRO infection (MDRINF>48h) (Figure 1). We compiled patient characteristics and outcomes (Table 1) overall and for no-MDR and MDR>48h patients, further stratified as MDRCOL>48h and MDRINF>48h. Median age was 65 years (IQR 59–71 years); 95/347 (27.4%) patients were female. More than 80% of patients had >1 concurrent condition, and hypertension was the most common (181/347, 52.2%). Patients who had ever smoked were more frequent in the MDRINF>48h group (26/100, 26%) than in the MDRCOL>48h group (11/107, 10.3%; p = 0.003). Transfer to the ICU occurred mostly from nonintensive hospital wards (159/347, 45.8%), but relevant proportions were transferred directly from the emergency department (102/347, 29.4%) or from other ICUs (86/347, 24.8%). Patients were transferred to ICU early during hospitalization, a median time of 5 days from first hospital admittance.
Groups did not differ for steroid use or antimicrobial drug therapies received before ICU admission. According to clinical practice, steroids had been administered for SARS-CoV-2 infection management in 252/347 (72.6%) patients, mostly (228/347, 65.7%) with only a standard dose (dexamethasone 6 mg/d). Most patients (221/347, 63.7%) had previously received antimicrobial drugs before ICU admission. MDRO events before ICU admission were reported in only 4 patients (1.2%). During ICU stay, 118 patients (34%) died, but there were no significant differences between groups. When compared with no-MDR patients, we found that MDR>48h patients had a longer duration of mechanical ventilation (median 18 vs. 14 days; p = 0.001) and of ICU stay (median 25 vs. 15.5 days; p = 0.001). Those differences were largely caused by the MDRINF>48h group (Table 1).
Bacterial Isolate Description and Incidence
Complete microbiologic reports were available for 426/435 patients, including 338/347 patients (97.4%) with no MDRO isolates within the first 48 hours of ICU admission. We describe the selection process conducted to assess incidences of HAIs and of MDRO events distinguishing between colonization and infection (Figure 2). We identified 801 bacterial isolates from 271 patients that correspond to first MDRO colonization (255 isolates in 173/338 patients, 51.2%) and new episodes of bacterial superinfections, either by MDRO (130 isolates in 95/338 patients, 28.1%) or antimicrobial drug‒susceptible bacteria (non-MDRO, 416 isolates in 212/338 patients, 62.7%). A total of 73 (21.6%) patients had MDRO colonization and MDRO infection develop during ICU stay, and infections were caused by the same colonizing bacterial species in nearly one third of them (24/73, 32.9%) (Appendix Table 3). Clinical interpretation of bacterial isolates as colonization/infection by attending physicians at the time of arrival of microbiologic results was found to be highly concordant with the retrospective evaluation conducted according to international guidelines (κ coefficient 0.902, 95% CI 0.890–0.913) (Appendix Table 4).
Overall, 546 bacterial HAIs were recorded, 130 (23.8%) caused by MDRO. Gram-negative bacteria accounted for 59.7% (326/546) of all HAIs and for 60% (78/130) of infections caused by MDROs. Bacterial species responsible for HAIs varied by infection site and severity of infection (Appendix Tables 5, 6). Ventilator-associated lower respiratory tract infections (VALRTIs) represented most infectious episodes (359/546, 65.7%), followed by BSI (141, 25.8%) and urinary tract infections (40, 7.3%). Among BSIs, 31/141 (22%) were associated with a central line, 43 (30.5%) were secondary to VALRTI or urinary tract infections, and the remaining 67 (47.5%) were classified as primary BSI without a known bacteremic focus (Appendix Figure 3).
Among MDRO colonization, Enterococcus faecium (112/255 isolates, 43.9%) was the most frequent isolate, followed by Klebsiella spp. (34, 13.3%), Escherichia coli (26, 10.2%), Staphylococcus aureus (25, 9.8%), Pseudomonas aeruginosa (15, 5.9%) and Acinetobacter baumannii (13, 5.1%). We compiled the percentages of MDRO colonization, MDRO HAIs, and non-MDRO HAIs for the most frequently isolated bacteria of the World Health Organization priority pathogens list (27) (Appendix Figure 4).
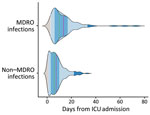
First MDRO colonization occurred at a median time of 13 (IQR 8–12) days after ICU admission. HAIs caused by antimicrobial drug‒susceptible bacteria occurred earlier than in those caused by MDROs at 6 (IQR 3–10) and 10 (IQR 6–17) days from admission (p<0.001) (Figure 3). The incidence rates for MDRO colonization was 29.97 cases/1,000 patient-days (95% CI 26.34–34.10), for MDRO infection was 14.99 cases/1,000 patient-days (95% CI 12.36–18.19), and for non-MDRO infection, was 50.12 cases/1,000 patient-days (95% CI 44.59–56.32). Infection rates varied substantially by infection site (Table 2).
Association of Antimicrobial Drugs and Steroids to MDRO Events
We investigated possible associations between MDRO events and previous steroid and antimicrobial drug therapies (Appendix Tables 7, 8). Because steroids were included in the management of COVID-19 pneumonia from the early stage of the disease, we evaluated their intake before and during ICU stay. Almost the entire population had received steroid therapy (313/338, 92.6%), without major differences between no-MDR (132/140, 94.3%), MDRCOL>48h (98/103, 95.1%) and MDRINF>48h (83/95, 87.4%) (Appendix Table 7).
To assess possible association between MDRO events and previous antimicrobial drug use, we focused on therapies administered during the first 10 days of ICU stay. This timeline was set to balance observation time between no-MDR and MDR>48h groups because three fourths of MDRO events occurred within this timeframe. Also, three fourths of patients in no-MDR group stayed in ICU >10 days (Appendix Table 8). Previous exposure to antimicrobial drugs was notably higher in patients who developed MDRO events than in patients who did not (116/197 [58.9%] in MDR>48h vs 18/140 [12.9%] in no-MDR; p<0.001) (Appendix Table 8).
Discussion
We describe incidences and clinical characteristics of HAIs and MDRO events, distinguishing between colonization and infection, in a large cohort of ICU COVID-19 patients from a country with high prevalence of MDRO (28). Despite being composed of patients admitted from >45 different hospitals, our cohort is homogeneous for concurrent conditions and risk factors for MDRO acquisition, clinical severity of COVID-19, management of antimicrobial drug therapy, and infection prevention and control strategies within the ICU, including surveillance sampling.
Antimicrobial drug resistance represents a major challenge in the ICU. Its occurrence is the result of the influx of previously colonized patients and acquisition of MDROs during ICU stay, as a consequence of antimicrobial drug overexposure and interpatient transmission, as well as contact with colonized healthcare workers, fomites, or the environment. The incidence of MDROs is strongly influenced by pandemic periods, such as during COVID-19, when unprecedented patient loads in ICUs resulted in breaches in IPC, such as gaps in microbiologic surveillance, lack of communication between clinicians, and reduced attention to environmental measures and contact precautions among healthcare workers (29). In addition, ICU admissions caused by viral pandemics place a strain on ICU resources, requiring the reallocation of non-ICU beds, along with the use of non-ICU staff to meet the urgent demand. In this setting, strengthening measures, such as active surveillance with prompt recognition of outbreaks, staff training, increased environmental disinfection and cohorting, become essential to reducing MDRO circulation (30).
In the pre–COVID-19 pandemic era, the prevalence of infections caused by MDROs in ICU patients varied from a reported rate of 14.1% in VALRTIs acquired in ICUs in North America (31) to an average of >40% in 2 large multicentric worldwide studies of nosocomial BSIs (32,33). Variability exists between participating countries, ranging from 8% (Australia) to >75%–80% in Asia, eastern Europe, and southern Europe. Carbapenem resistance was present in more than one third of gram-negative bacteria, and 36% of all gram-positive bacteria were MDR (32,33).
Several studies have been published on MDRO incidence, etiology and source of HAIs in ICU COVID-19 patients (4–21) (Table 3). Most of those studies evaluated overall MDRO infections or specific HAIs, such as BSI or VALRTIs (7,15–17,19,21), whereas colonization events were assessed in only a few studies (8–12,14). Incidence measures of MDRO events varied widely; cumulative incidence of the first MDRO event was 5%–57% (7,17) and incidence rate 2.6–31.48 cases/1,000 patient-days (11,16). The percentage of MDRO was 27%–100% for all recorded events (15,17). Compared with the amount of literature evaluating MDRO events during ICU stay, we found that few data are available on MDRO proportions among COVID-19 patients at ICU admission. In recent work of the multicenter HAI-ICU surveillance network in France, the percentage of MDR gram-negative bacteria among >4,000 COVID-19 patients admitted was 11.7% (34).
In our cohort, 20% of patients had MDRO isolation within the first 48 hours, indicating acquisition before ICU admittance. We found that patients who had MDROs isolated during the first 48 hours were more frequently transferred from other ICUs and exposed to a higher number of antimicrobial drugs before ICU admission. Both of those factors are well known to be associated with development of infections by antimicrobial drug‒resistant pathogens (6). Only 2.7% of our cohort had MDRO colonization/infection before ICU admission. The marked difference between expected and observed MDRO prevalence at ICU admission probably reflects the major issues in IPC during the emergency situation of the pandemic.
Considering patients without MDRO isolation within the first 48 hours, we observed no differences in demographic characteristics or in clinical severity at admission between patients who showed or not showed development of MDRO events during ICU stay, underlying consistency between groups at ICU admission. In our cohort, we did not find direct association between MDRO infection and in-ICU deaths. However, length of ICU stay and duration of mechanical ventilation were longer for patients with MDRO events and, among them, longer for patients who had infections than for colonized patients. No causative effect can be drawn from these results because occurrence of MDRO events could be either responsible for longer ICU stay or its direct consequence because of longer exposure time (35,36).
Active surveillance screening coupled with the evaluation of all microbial isolates enabled us to precisely identify patients who had with MDRO events. Two thirds of the cohort showed development of MDRO colonization or infection during ICU stay. Half of our patients were given diagnoses of MDRO colonization during ICU stay, compared with 21% observed in a recent study analyzing a smaller population (10). Our results can be, in part, explained by strict routine microbiologic surveillance, which enabled prompt and precise recognition of such cases. Data from previous studies on bacterial superinfections in COVID-19 ICU patients are heterogenous and describe MDRO HAIs in 11%‒50% of the population (6,13). Our results confirm the substantial risk for mechanically ventilated COVID-19 patients to have MDRO infections develop; such infections affected almost 30% of our cohort during ICU stays. Also, more than twice as many patients had antimicrobial drug‒susceptible HAIs.
We found high concordance between clinical diagnosis and retrospective evaluation of HAIs according to literature criteria. We believe this result well demonstrates how implementation of structured antimicrobial stewardship and IPC measures, with collaboration of infectious disease consultants and intensivists, can strongly effect management of critically ill patients, favoring accurate diagnosis and therapeutic choices, according to international guidelines.
Patients who had MDRO events had greater exposure to antimicrobial drugs the first 10 days of ICU stay than patients who had no MDRO findings. This observation is consistent with results of recent studies conducted on large population of patients, which showed major associations between exposure to specific antimicrobial drug classes and drug resistance, and a decreasing pattern over time (37,38). However, accurate analysis of the association between antimicrobial drug exposure and MDRO events was beyond the scope of this study because other variables, such as average intake time of each antimicrobial drug class and infections with antimicrobial drug‒susceptible bacteria during the observation time, should be considered.
The first limitation of this study is that it was a retrospective monocentric cohort and, therefore, had intrinsic risks of limited accuracy and generalizability. However, interpretation of all microbiologic findings has been conducted ex post on the basis of standardized literature criteria and independent from the physicians' view. Also, even though the study was monocentric y, patients were admitted from >45 hospitals and assisted by different hospital staff. Advantages to this study design derive from the standardized microbiologic surveillance, both in terms of timing and laboratory method, as well as from homogeneous antimicrobial stewardship and IPC strategies among ICU modules. This factor enabled us to provide precise and consistent data in terms of incidence of HAIs and MDRO events, not only infections but also colonization.
Second, this study was not conducted for evaluation of the effect of antimicrobial drugs on development of MDRO or the effect of MDRO events on ICU deaths and length of stay; the sample size was probably inadequate for these issues. Therefore, our findings on this issue should be interpreted with caution.
Third, patients' data before ICU admission were retrieved from information registered at ICU entry and not from hospital databases of the single referring centers. Accuracy of previous MDRO events and steroids and antimicrobial drug treatments might be limited, although these factors play a major role in routine management of ICU patients, and we do not expect major gaps in data acquisition.
In conclusion, our in-depth analysis of incidence measures of HAIs and MDRO events contributes to increase knowledge of MDRO colonization and infections in ICU COVID-19 patients. These findings should be a priority in contributing toward IPC and antimicrobial stewardship policies for ensuring the best clinical care.

Comments
Post a Comment