Pathogenic Bacteria and their Antibiotic Resistance Patterns | IDR - Dove Medical Press
Tsegahun Asfaw,1 Deribew Genetu,2 Demissew Shenkute,1 Tassew Tefera Shenkutie,1 Yosef Eshetie Amare,3 Habtemariam Alekaw Habteweld,4 Berhanu Yitayew1
1Department of Medical Laboratory Science, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Medical Laboratory Science, Injibara University, Injibara, Ethiopia; 3Departments of Biomedical Science, Debre Berhan University, Debre Berhan, Ethiopia; 4Departments of Pharmacy, Debre Berhan University, Debre Berhan, Ethiopia
Background: Bacterial contamination of milk is a primary culprit for causing foodborne illnesses, presenting a significant health hazard for millions of individuals around the globe. The level and variety of microorganisms present in raw milk determine its degree of contamination and the potential health risks it poses.
Methods: A cross-sectional survey was conducted from February to August. A questionnaire was used to collect data on socio-demographic characteristics and hygiene practices from milk distributors and traders. Raw milk, yoghurt, swabs from milk containers and drinking cups were collected and processed for bacterial isolation and identification, antibiotic susceptibility testing, MDR screening and confirmation, ESBL screening and confirmation. Finally, all data were pooled and analyzed using SPSS software version 25.
Results: A total of 120 samples of fresh milk, yogurt and cotton swabs from milk containers and cups were collected. A total of 80 bacterial isolates were isolated from 120 samples. Among the bacteria isolated, S. aureus 17 (21.3%), E. coli 17 (21.3%), S. epidermidis 14 (17.5%), Klebsiella spp. 9 (11.3%) and Salmonella spp. 7 (8.8%) were detected most often. High rate of contamination was observed in fresh milk 23 (28.8%) and yogurt 23 (28.8%). All isolates were resistant to at least one antibiotic tested. Comparatively, high rates of resistance were observed in all isolates to the most commonly prescribed antibiotics in Ethiopia. However, lower rates of resistance have been observed for recently introduced antibiotics in Ethiopia. Of the isolates, 20 (25.0%) were resistant to eight or more antibiotics. While 16 (20.0%), 12 (15.0%), 9 (11.3%) isolates were resistant to two, three and five antibiotics, respectively. Of the bacteria isolated, 52/80 (65.0%) were MDR, 25/49 (51.0%) were screened for ESBL production, and 20/49 (40.8%) isolates were confirmed as ESBL producer.
Conclusion: This study showed a high rate of bacterial isolates along with MDR and ESBL-producing strains in raw milk, yoghurt, milk container swabs and drinking cup swab samples, associated with poor hygiene and sanitation practices.
Keywords: bacterial contamination, multidrug-resistance, extended-spectrum beta-lactamase, raw milk, yoghurt, milk contact surface
Introduction
The human burden of food-borne disease is still poorly understood.1 Over the past decade, most countries have seen a significant increase in the incidence of food-borne disease.2 Dairy products such as milk and yogurt, which are common foods in many countries, provide a favourable environment for the growth of many microorganisms due to their nutritional content.3 Many studies have been conducted to improve raw milk quality, reduce the risk of microbial contamination, and improve the chemical and nutritional quality of dairy products.3–5 Today, daily consumption of milk and dairy products is becoming increasingly popular due to potential benefits such as rich nutrients, beneficial bacteria, and prevention of lactose intolerance are increasingly welcomed. However, due to the possible presence of pathogens and their toxins, consumption of raw milk can pose a significant risk of food-borne disease.6–9
The dairy products that are produced from raw milk are often found to contain Staphylococcus aureus, Salmonella spp., Listeria monocytogenes, and E. coli, which are among the most common pathogens.4,8 In addition, Staphylococcus aureus, Listeria monocytogenes, and Salmonella spp. may contribute to bovine mastitis and can be directly excreted in milk.8–11 According to the World Health Organization (WHO), foodborne pathogens were responsible for approximately 600,652,361 cases and 418,608 deaths worldwide in 2010.12 The highest burden of foodborne illness per capita has been reported in Africa, with a median foodborne disability-adjusted life-year (DALY) of 2455 per 100,000 populations.13 Among these cases, 26.6% were attributed to Salmonella spp., 11.2% to Enteropathogenic E. coli, 8.6% to Enterotoxigenic E. coli, 0.08% to Listeria monocytogenes, 5.7% to Campylobacter spp., and 0.004% to Shiga-toxin-producing E. coli.12,13
In developing countries, especially Ethiopia, milk is a major cause of food-borne disease. This happens when milk and various dairy products are produced under unsanitary conditions and poor production practices.14 Although contamination control of raw milk and dairy products is not routinely practiced, the Ethiopian dairy industry is evolving towards a market-based system.15 A survey conducted in central Ethiopia found that 31.8% of the farmers consumed raw milk.16 In the dairy market value chain, unsanitarily processed milk is easily contaminated by milk-borne bacterial pathogens, making it a convenient carrier for disease transmission and posing a significant public health risk to consumers.17–19 A study conducted in northern Ethiopia found that milk contamination rates ranged from 45% to 75%.20
Overdose, misuse and long-term use of pharmaceuticals to treat animals and humans have led to alarming growth and prevalence of antibiotic-resistant bacteria. This exacerbates the clinical situation and poses one of the greatest medical challenges of our time, contributing to poor cure rates, loss of human and animal life, and animal dairy products.21 Therefore, this study was designed to assess bacterial milk contamination, and the resistance patterns of bacterial isolated from raw milk, yoghurt, and contact surfaces in Debre Berhan Town, Ethiopia.
Methods
Study Design and Area
The cross-sectional survey was conducted from February to August 2022 in the town of Debre Berhan, 130 km northeast of Addis Ababa. In the town, consumers brought most of their milk and dairy products directly from farmers, traders/traders and cafeterias. People in the town of Debre Berhan and the surrounding villages also regularly consume milk and dairy products.
Data and Sample Collection
A questionnaire was used to collect data on socio-demographic characteristics and hygiene practices from vendors and milk handlers. Data on facility sanitary conditions were collected through individual interviews and observations. Four types of samples were collected: fresh milk, yoghurt, milk container cotton swabs, and drink cup cotton swabs. An equal number of samples (30 each, 120 total) were randomly collected from farmers, vendors, or cafeterias. Fifty millilitres of raw milk and yogurt were collected. Environmental swab samples from milk containers and drinking cups were collected over an area of 30 cm2 by using cotton swabs soaked with sterile buffered peptone water (BPW).
Sample Processing, Isolation and Identification of Bacteria
Approximately 1 mL of raw milk and yogurt samples were transferred to sterile test tubes containing 9 mL of BPW. Cotton swab samples from the milk container and drinking cup were placed in a sterile test tube and suspended in a test tube containing 9 mL of BPW. All samples were labelled, placed in sterile plastic bags and transported to Debre Berhan University Microbiology Laboratory. The mixture was then serially diluted. Finally, 0.1 mL volumes of diluted samples were aseptically taken and inoculated on solidified MacConkey (Oxoid Ltd., Basingstoke and Hampshire, UK) and mannitol salt agar (Oxoid Ltd.) using the pour plate method. After pure colonies were obtained and key characteristics were recorded, the isolated organisms were further identified using a series of biochemical tests. Gram-negative bacteria were identified based on colonial morphology and pigmentation, oxidase test, carbohydrate fermentation, H2S production, citrate utilization, motility, growth at 42°C, indole formation, lysine decarboxylase and lysine deaminase production, and urea hydrolysis. Gram-positive isolates were also differentiated by colonial characteristics, catalase test coagulase tests, and novobiocin susceptibility test.
Antimicrobial Susceptibility Testing
Antimicrobial resistance profiles of the isolates were determined using the standard Kirby–Bauer disk diffusion method described by CLSI-2022.21 Bacterial cultures were prepared by suspending freshly cultured bacteria in 4–5 mL of sterile saline and adjusting the turbidity to the McFarland standard turbidity of 0.5. After standardizing the bacterial suspension, a sterile cotton swab was soaked and twisted several times with firm pressure against the inner wall of the tube to remove excess liquid. The dry surface of Mueller Hinton agar plates (Oxoid Ltd.) was inoculated by spreading a cotton swab across the surface. The antibiotic disc was then placed onto the inoculation plate using sterile forceps and incubated overnight (18–24 hours) at 37°C. Bacterial isolates were tested for the antibiotics commonly prescribed in Ethiopia, in accordance with the Ethiopian Ministry of Health Antimicrobial Prescribing Policy. The antibiotics tested were amoxicillin (AMC, 30μg), ampicillin (AMP, 10μg), penicillin (P, 10μg), cotrimoxazole (SXT, 30μg), ciprofloxacin (CIP, 5μg), chloramphenicol (CAF, 30μg), gentamicin (CN, 10μg), erythromycin (E, 15μg), tetracycline (TC, 30μg), doxycycline (DXT, 30μg), methicillin (MET, 5 µg), ceftriaxone (CRO, 30μg), imipenem (IMI, 10μg), meropenem (MRP, 10μg), cefotaxime (CTX, 30μg), and ceftazidime (CAZ, 30μg).
Multidrug-Resistant Isolates
Bacterial strains resistant to one or more antibiotics from three or more antibiotic classes are considered multidrug resistant.22
Confirmation of ESBLs-Producing Bacteria
Enterobacteriaceae isolates with reduced susceptibility and resistance to cefotaxime and/or ceftazidime were included as potential ESBL producers. Isolates with a ceftazidime (30 μg) zone of inhibition size of ≤22 mm and/or a cefotaxime (30 μg) zone of inhibition size of ≤27 mm were considered potential ESBL producers.21 To confirm ESBL production, ceftazidime (30 μg) and cefotaxime (30 μg) discs alone and in combination with clavulanic acid (30 μg/10 μg) were placed 25 mm centre to centre on Mueller–Hinton agar overlaid with the bacterial suspension and incubated overnight (18–24 hours) at 37 °C. Bacterial isolates were identified as ESBL producers that increased the zone of inhibition diameter of the combined discs by more than 5 mm compared to ceftazidime or cefotaxime discs alone.21
Quality Control
Prior to the actual work, reagents were checked for proper functioning and handled according to standard procedures. Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used as quality control organisms throughout the antimicrobial susceptibility testing. For ESBLs confirmatory test, ESBLs positive K. pneumoniae ATCC 700603 and ESBLs negative E. coli ATCC 25922 control strains were used.
Statistical Analysis
Data obtained from questionnaires and laboratory procedures were summarized and analyzed using SPSS software version 25. Cross tabulation was performed, and quantitative values (frequency and percentage) are shown in the statistical table.
Result
Socio-Demographic and Hygienic Practice of the Study Participant
All milk and swabs of milk contact surfaces were collected from farmers (5, 16.7%), vendors (8, 26.7%) and cafeterias (17, 56.7%). All persons working on milk business (100%) did not have any formal training on milk handling and marketing. Majority of the business owners 22 (73.3%) had a habit of mixing milk from different farms or sources. Most of the business owners 15 (50.0%) waited less than 1 hour to received their milk, but milk for sale was held back for 1–2 hours by 11 (36.7%) of the milk business owner. Similarly, 17 (56.7%) of the business owners took around a day to complete the milk. Most of the correspondents clean the milk containers and utensil daily 23 (76.7%) with hot water with detergent/soap 16 (53.3%) (Table 1).
 | Table 1 Socio-Demographic, Hygienic Practice of Vendors and Cafeteria at Debre Berhan Town, Ethiopia, 2022 |
Bacterial Contamination of Milk, Yoghurt and Milk Contact Surfaces
A total of 80 bacteria were isolated from 120 samples. Of the isolates, 31 (38.8%) were Gram-positive and 49 (61.2%) were Gram-negative. Among the nine different bacterial species isolated, S. aureus 17 (21.3%), E. coli 17 (21.3%), S. epidermis 14 (17.5%), Klebsiella spp. 9 (11.3%) and Salmonella spp. 7 (8.8%) were most frequently detected (Table 2).
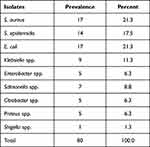 | Table 2 Prevalence of Bacterial Contamination in Milk, Yoghurt and Milk Contact Surfaces at Debre Berhan Town, Ethiopia, 2022 |
The rate of contamination was high in raw milk (23, 28.8%) and yoghurt (23, 28.8%). Among gram-positive bacteria species, S. aureus was the predominant isolate in raw milk (4, 23.5%), yoghurt (5, 29.4%), and milk container swabs (5, 29.4%). S. epidermidis was also the predominant isolate from milk container swabs (5, 35.7%) and drinking cup swabs (7, 50.0%). E. coli was found to be the most common enteric bacterium in raw milk (8, 57.1%). The same is true for Salmonella spp. (4, 57.1%). Other pathogens, such as Klebsiella spp., Enterobacter spp., Citrobacter spp., Proteus spp., and Shigella spp. were also isolated from milk, yoghurt and milk contact surfaces (Table 3).
 | Table 3 Distribution of Bacterial Contamination in Milk, Yoghurt and Milk Contact Surfaces at Debre Berhan Town, Ethiopia, 2022. |
Antibiotic Resistance Patterns of Isolates in Milk, Yoghurt and Milk Contact Surfaces
The highest level of resistance was observed in ampicillin (79, 98.8%), amoxicillin (75, 93.8%), and penicillin (30, 96.8%). For example, all isolates except S. epidermidis (13, 92.9%) were 100% resistant for ampicillin. Also, the most isolated bacterial species like S. epidermidis (14, 100.0%), Salmonella spp. (7, 100.0%), Citrobacter spp. (5, 100.0%), Shigella spp. (5, 100.0%), S. aureus (16, 94.1%), E. coli (16, 94.1%) and Klebsiella spp. (8, 88.9%) were the most resistant pathogen against amoxicillin. Gram-positive bacteria also showed the highest level of resistance for penicillin (30, 96.8%) (Table 4).
 | Table 4 Antibiotics Resistance Patterns of Bacterial Contamination in Milk, Yoghurt and Milk Contact Surfaces at Debre Berhan Town, Ethiopia, 2022. |
Generally, high rate of resistance was observed in all isolates for the most prescribed antibiotics in Ethiopia. For example, (53, 66.3%), for erythromycin, (46, 57.5%), for cotrimoxazole, (47, 58.8%) for doxycycline, (42, 52.5%) for ceftriaxone, (40, 50.0%) for gentamycin and (45, 56.3%) for chloramphenicol a high rate of resistance was observed. However, lower resistance rate was observed for lately introduced antibiotics to Ethiopia like meropenem (16, 20.0%), imipenem (19, 23.8%), ceftazidime (24, 30.0%) and ceftaxime (27, 33.8%) (Table 4).
Among the isolates, 20 (25.0%) were resistant for eight and more antibiotics (eg, S. aureus (5, 29.4%), E. coli (5, 29.4%), S. epidermidis (4, 23.5%), and Enterobacter spp. (2, 40.0%)), while 16 (20.0%), 12 (15.0%), and 9 (11.3%) of the isolates were resistant for two, three, and five, antibiotics, respectively (Table 5).
 | Table 5 Multiple Drug Resistance Patterns of Bacterial Contamination in Milk, Yoghurt and Milk Contact Surfaces at Debre Berhan Town, Ethiopia, 2022. |
Multiple Drug-Resistant and ESBL-Producing Bacterial Isolates in Milk, Yoghurt and Milk Contact Surfaces
Among isolated bacteria, 52/80 (65.0%) were MDR, 25/49 (51.0%) were screened for ESBL production and 20/49 (40.8%) isolates were confirmed as ESBL producer. Citrobacter spp. was 100% MDR followed by Enterobacter spp (4, 80.0%), S. epidermidis (10, 71.4%), Klebsiella spp. (6, 66.7%), E. coli (11, 64.7%), and S. aureus (11, 64.7%). Majority of the isolates, like Enterobacter spp. (4, 80.0%), Citrobacter spp. (3 60.0%), E. coli (10, 58.8%) and Klebsiella spp. (4, 44.4%), were screened for ESBL production. Enterobacter spp. (4, 80.0%), E. coli (8, 47.1%), Klebsiella spp. (4, 44.4%), and Citrobacter spp. (2, 40.0%), were among the isolates confirmed for ESBL production (Table 6).
 | Table 6 Distribution of MDR and ESBL Confirmed Bacterial Contamination in Milk, Yoghurt and Milk Contact Surfaces, at Debre Berhan Town, Ethiopia, 2022. |
Discussion
A total of 80 bacteria of 9 different species were isolated from a total of 120 samples (milk, yoghurt and milk contact surfaces). Of the isolates, 31 (38.8%) were gram-positive and 49 (61.2%) were gram-negative. Similar studies in Ethiopia16,19,23,24 also isolated many different bacteria in milk. The high prevalence of bacterial species in this study was associated with commercial use of unpasteurized milk, suboptimal hygiene practices, inadequate refrigeration, and lack of appropriate equipment suitable for storing and transporting milk. Microbial contamination in the milk market value chain can be caused by diseased cows, unhygienic milking practices, poor personal hygiene, unhygienic milking utensils and/or equipment, and poor preservation and inadequate supply of drinking water.14,25–27 In developing countries like Ethiopia, many dairy farmers do not disinfect the nipples of the cow before milking and wash their hands poorly before milking. Poor hygienic practices and lack of standard milking procedures have been reported throughout the milk value chain system in Ethiopia.14
In this study, the S. aureus contamination level was 17 (21.3%). This finding is comparable to studies in Tigray, Ethiopia23 and Sebeta, Central Oromia,24 but lower than another study in Tigray, Ethiopia.19 Similarly, this study documented lower levels of contamination compared to the report from Côte d'Ivoire.28 Furthermore, this study reported a lower rate of S. aureus milk contamination than the study in Madurai, South India.29 High levels of S. aureus infection indicate unhygienic handling and milking procedures. This was further concluded to be due to the predominant presence of S. aureus in raw milk (4, 23.5%), yoghurt (5, 29.4%) and milk container swabs (5, 29.4%). The overall contamination rate for S. epidermidis was 14 (17.5%). In addition, it was the predominant isolate from milk container swabs (5, 35.7%) and drinking cup swabs (7, 50.0%). This indicates cross-contamination via human skin, as S. epidermidis is one of the most common bacterial colonizers of healthy human skin.
This study showed that intestinal bacteria such as E. coli (17, 21.3%), Salmonella spp. (7, 8.8%) and Klebsiella spp. (9, 11.3%) were detected most often. Previous studies have also shown that coliform bacteria are most common in milk and milk containers because they are abundant in animals and in the environment.30–32 E. coli and Salmonella spp. presents the main microbiological risks associated with the consumption of dairy products made from raw milk or cow's milk contaminated after pasteurization, mainly in developing countries where hygiene standards are low.33 Overall, this study reports that milk and dairy products are susceptible to many pathogens, including Salmonella spp., S. aureus and Enterobacter spp., E. coli and Klebsiella spp., and Citrobacter spp. Many factors contribute to the prevalence and presence of pathogens in milk and its products, including farm size, number of livestock, milking hygiene, farm management practices, environmental sanitation for processing, post-processing, and transportation as well as geographical location and season.9,34–37
Milk and dairy products are contaminated with AMR pathogens from a variety of sources that pose a risk of contamination, including the animals themselves, dirty milk containers, milk handlers, airborne dust and droplets during production and processing.37 Therefore, it is very important to minimize the emergence and spread of multidrug-resistant microorganisms that can be transmitted from animals or animal products such as milk and meat, and to maintain the effectiveness of currently available antibiotics.
In this study, the overall resistance of isolates was observed for ampicillin (79, 98.8%), amoxicillin (75, 93.8%) and penicillin (30, 96.8%). For example, S. aureus were 100% and 16 (94.1) resistant to ampicillin and penicillin, respectively. Another study also reported the same result for specific bacterial species. For example, a study in Bishoftu, Ethiopia37 and South Africa38 reported 100% penicillin resistance rate. S. epidermidis also showed 100% and 13 (92.9%) resistance against amoxicillin, penicillin and ampicillin, respectively. Most of the isolates of intestinal bacteria such as Salmonella spp., Citrobacter spp., Shigella spp. also showed 100% resistance to amoxicillin, while E. coli and Klebsiella spp. were 16 (94.1%) and 8 (88.9%) resistant to amoxicillin, respectively. This may be because these drugs are easily available at low cost leading to abuse and misuse.
This study found relatively high rates of resistance to Ethiopia's most commonly prescribed antibiotics. Examples: 53 (66.3%) on erythromycin, 46 (57.5%) on co-trimoxazole, 47 (58.8%) on doxycycline, 42 (52.5%) on ceftriaxone, 40 (50.0%) on gentamicin and 45 (56.3%) on chloramphenicol were observed. Many studies in Ethiopia and abroad have also reported high levels of individual species resistance to these antibiotics.39–46 This might be due to over-prescription of antibiotics, under-administration by patients, over-use of antibiotics in livestock and farmland, poor infection control in health services, and poor sanitation and hygiene facilities.
Results of this study showed that 58/80 (72.5%) of the isolates were resistant to three or more antibiotics (eg, S. aureus 13/17 (76.5%), E. coli 13/17 (76.5%), S. epidermidis 11/14 (78.6%), Citrobacter spp. 100%, Klebsiella spp. 6/9 (66.7%), Salmonella spp. 4/7 (57.1%), Enterobacter spp. 4/5 (80.0%)). A study conducted in Ethiopia reported higher results than our results for S. aureus. For example, a study in Bishoftu,37 Haramaya47 and Adama48 reported resistance rates of S. aureus of 98.39%, 87.6% and 94.4%, respectively. However, another study conducted in Addis Ababa, Ethiopia49 and Brazil,50 reported 45.1% and 64.4% S. aureus resistant to three or more antibiotics, respectively. This study also reported a lower rate of resistance (to three or more antibiotics) for E. coli (76.5%) than the study conducted in Bishoftu town, Ethiopia51 reported 92.5%. In addition, studies in South Africa52 reported at least three types of antibiotic-resistant E. coli. In the present study, Salmonella spp. was found to be 57.1% resistant to three or more antibiotics, a comparable study in Bishoftu, Ethiopia37 and higher than the study in Addis Ababa, Ethiopia46 reported 53.85% and 50%, respectively. But studies in Jimma town, Ethiopia53 and Kersa District, Jimma Zone, Ethiopia,54 reported rates of 83.3% and 70%, respectively. According to this study, with the exception of recently introduced antibiotics in Ethiopia such as meropenem, cefotaxime and ceftazidime, all tested antibiotics were the most frequently observed patterns for most bacteria tested.
Of the 80 bacteria isolated, 52/80 (65.0%) were MDRs and 20/49 (40.8%) isolates were confirmed as ESBL producers. Previous studies conducted in different parts of the world reported increased levels of MDR5,55–64 and ESBL-producing bacteria.65–69 The higher levels of resistance observed in this study may be due to inappropriate antibiotic use on dairy farms. The results show that the use of these antibiotics is common in the study area. Significant levels of resistance to many drugs pose a public health risk because foodborne outbreaks are difficult to treat, and this group of MDRs in the food supply is a reservoir for resistance genes that can spread. Due to the relatively limited availability and high cost of newly developed drugs, reports of antibiotic resistance rates for relatively cheap and frequently available antibiotics are alarming for low-income communities living in developing countries, such as Ethiopia.
Limitations of the Study
Identification of diarrheagenic Escherichia coli strains, enterotoxic strains of Staphylococcus aureus, and species identification of the majority of bacterial isolates was not conducted. Furthermore, clonal relationships of the isolates and molecular characterization of multidrug-resistant and extended-spectrum beta-lactamase-positive isolates was not performed. Owing to the limited sample size, statistical associations that could elucidate the correlation between milk processor hygiene practices and multidrug-resistant and extended-spectrum beta-lactamase-positive isolates were not demonstrated.
Conclusion and Recommendation
The levels of milk contamination in this study were high, suggesting the existence of significant health risks to consumers. Milk collected from farmers, milk vendors and cafeterias was significantly associated with higher levels of milk contamination. Milk distributors and cafe owners should apply good hygiene and hygiene practices when handling milk. Use suitable, clean cold chains and containers during transportation; and refrigerate the milk during storage. Government agencies should establish quality and safety standards for commercially produced milk to improve microbiological quality and milk safety.
In this study, the presence of resistant pathogenic bacteria on milk, yogurt, milk containers and drinking cups indicated poor hygiene, which is a major health concern for consumers. Existing research has also clearly shown that enteric bacteria such as E. coli and Salmonella spp. isolated from human and animal feces can contaminate milk and milk containers due to poor hygiene standards. In addition, high levels of MDR and ESBL-producing bacteria were detected in milk and milk contact surfaces; it also revealed evidence of inappropriate antibiotic use in animals and humans. Therefore, an ongoing program of resistance surveillance should be implemented nationwide.
Ethical Consideration
Ethical approval was obtained from the Debre Brehan University Institutional Review Board [Protocol Number: IRB-003], and formal support was obtained from the Debre Brehan Town, North Shoa Zonal Office. All participants were informed of the purpose of the study. Ultimately, verbal and written consent was obtained from each milk handlers, trader and distributor.
Disclosure
The authors declare that this study was conducted in the absence of any commercial or financial relationships that would be considered a potential conflict of interest.
References
1. Newell DG, Koopmans M, Verhoef L, et al. Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol. 2010;139:S3–S15. doi:10.1016/j.ijfoodmicro.2010.01.021
2. World Health Organization. General information related to microbiological risks in food; 2012. Available from: http://www.who.int/foodsafety/micro/general/en/index.html.
3. Moosavy MH, Kordasht HK, Khatibi SA, Sohrabi H. Assessment of the chemical adulteration and hygienic quality of raw cow milk in the northwest of Iran. Qual Assur Safety Crops Foods. 2019;11(5):491–498. doi:10.3920/QAS2019.1605
4. Moosavi MH, Mahmoudi R, Ghorbanpour E, Khatibi SA. Evaluation of microbial and physicochemical characteristics of raw cow milk delivered to pasteurized milk plants in Tabriz city. Iran J Food Res. 2018;28(1):183–196.
5. Hassani S, Moosavy MH, Gharajalar SN, Khatibi SA, Hajibemani A, Barabadi Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci Rep. 2022;12(1):3878. doi:10.1038/s41598-022-07845-6
6. Heidinger JC, Winter CK, Cullor JS. Quantitative microbial risk assessment for Staphylococcus aureus and Staphylococcus enterotoxin in raw milk. J Food Prot. 2009;72(8):1641–1653. doi:10.4315/0362-028X-72.8.1641
7. Moosavy MH, Hallaj Salahipor M, Mostafavi E, Khatibi SA. Risk factors for human brucellosis in Mianeh. Iran J Zoonotic Dis. 2018;3(1):10–21.
8. Sugrue I, Tobin C, Ross RP, Stanton C, Hill C. Foodborne pathogens and zoonotic diseases. In: Raw Milk. Academic Press; 2019:259–272.
9. Oliver SP, Jayarao BM, Almeida RA. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog Dis. 2005;2(2):115–129. doi:10.1089/fpd.2005.2.115
10. Ding T, Suo Y, Zhang Z, et al. A multiplex RT-PCR assay for S. aureus, L. monocytogenes, and Salmonella spp. detection in raw milk with pre-enrichment. Front Microbiol. 2017;8:989. doi:10.3389/fmicb.2017.00989
11. Cobirka M, Tancin V, Slama P. Epidemiology and classification of mastitis. Animals. 2020;10(12):2212.
12. Havelaar AH, Kirk MD, Torgerson PR, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923. doi:10.1371/journal.pmed.1001923
13. World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases 2015. World Health Organization; 2015.
14. Yilma Z, Loiseau G, Faye B. Manufacturing efficiencies and microbial properties of butter and Ayib-Ethiopian cottage cheese. Livestock Res Rural Dev. 2007;19(7):1–12.
15. Alehegn W. Bacteriological Quality of Bovine Milk in Small Holder Dairy Farms in Debre Zeit, Ethiopia [M. Sc. thesis]. Addis Ababa, Ethiopia: Addis Ababa University; 2004.
16. Tarekgne E, Skeie S, Rudi K, Skjerdal T, Narvhus JA. Staphylococcus aureus and other Staphylococcus species in milk and milk products from Tigray region, Northern Ethiopia. Afr J Food Sci. 2015;9(12):567–576. doi:10.5897/AJFS2015.1373
17. Makita K, Desissa F, Teklu A, Zewde G, Grace D. Risk assessment of staphylococcal poisoning due to consumption of informally-marketed milk and home-made yoghurt in Debre Zeit, Ethiopia. Int J Food Microbiol. 2012;153(1–2):135–141. doi:10.1016/j.ijfoodmicro.2011.10.028
18. Angulo FJ, LeJeune JT, Rajala-Schultz PJ. Unpasteurized milk: a continued public health threat. Clin Infect Dis. 2009;48(1):93–100. doi:10.1086/595007
19. Reda M, Taddele H, Afera B, Bsrat A. Bacteriological quality assessment of milk in dairy farms, cafeterias and wholesalers in Adigrat, Tigray, Ethiopia. Eur J Biol Sci. 2014;6(4):88–94.
20. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340(2):c2096–c2096. doi:10.1136/bmj.c2096
21. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. In: CLSI Supplements M100S.
22. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
23. Berhe G, Wasihun AG, Kassaye E, et al. Milk-borne bacterial health hazards in milk produced for commercial purpose in Tigray, northern Ethiopia. BMC Public Health. 2020;20(1):894. doi:10.1186/s12889-020-09016-64
24. Ayele Y, Gutema FD, Edao BM, et al. Assessment of Staphylococcus aureus along milk value chain and its public health importance in Sebeta, central Oromia, Ethiopia. BMC Microbiol. 2017;17(1):1–7. doi:10.1186/s12866-017-1048-9
25. Kilango K. Food Safety in Milk Markets of Smallholder Farmers in Tanzania: A Case of Peri-Urban Wards in Temeke Municipality [Doctoral dissertation]. Sokoine University of Agriculture; 2011.
26. Lubote R, Shahada F, Matemu A. Prevalence of Salmonella spp. and Escherichia coli in raw milk value chain in Arusha, Tanzania. Am J Res Commun. 2014;2(9):1–13.
27. Khan AA, Massod FA, Bhat BA. Bacteriological quality and safety of raw milk in Kashmir valley. Wayamba J Animal Sci. 2011;3:2102–5789.
28. Kouamé-Sina SM, Makita K, Costard S, et al. Hazard identification and exposure assessment for bacterial risk assessment of informally marketed milk in Abidjan, Côte d'Ivoire. Food Nutr Bull. 2012;33(4):223–234. doi:10.1177/156482651203300402
29. Lingathurai S, Vellathurai P. Bacteriological quality and safety of raw cow milk in Madurai, South India. Webmed Central Microbiol. 2010;1(10):WMC001029.
30. Pantoja JCF, Reinemann DJ, Ruegg PL. Factors associated with coliform count in unpasteurized bulk milk. J Dairy Sci. 2011;94(6):2680–2691. doi:10.3168/jds.2010-3721
31. Stewart CM. Staphylococcus aureus and staphylococcal enterotoxins. In: Foodborne Microorganisms of Public Health Significance.
32. World Health Organization (WHO). WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Geneva, Switzerland: World Health Organization; 2015.
33. Mazurek J, Salehi E, Propes D, et al. A multistate outbreak of Salmonella enterica serotype Typhimurium infection linked to raw milk consumption—Ohio, 2003. J Food Prot. 2004;67(10):2165–2170. doi:10.4315/0362-028X-67.10.2165
34. Farrokh C, Jordan K, Auvray F, et al. Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production.. Int J Food Microbiol. 2013;162(2):190–212. doi:10.1016/j.ijfoodmicro.2012.08.008
35. Afroz H, Sultana F, Fakruddin M, Khan M, Uddin Z, Datta S. Isolation of Escherichia coli and Staphylococcus aureus from full cream powder milk sold under market conditions at Dhaka, Bangladesh and their antibiotic susceptibility. J Adv Sci Res. 2013;4:27–31.
36. Jayarao BM, Donaldson SC, Straley BA, Sawant AA, Hegde NV, Brown JL. A survey of foodborne pathogens in bulk tank milk and raw milk consumption among farm families in Pennsylvania. J Dairy Sci. 2006;89(7):2451–2458. doi:10.3168/jds.S0022-0302(06)72318-9
37. Geletu US, Usmael MA, Ibrahim AM, Di Cerbo A. Isolation, identification, and susceptibility profile of E. coli, Salmonella, and S. aureus in dairy farm and their public health implication in Central Ethiopia. Vet Med Int. 2022;2022:1–13. doi:10.1155/2022/1887977
38. Ateba CN, Mbewe M, Moneoang MS, Bezuidenhout CC. Antibiotic-resistant Staphylococcus aureus isolated from milk in the Mafikeng Area, North West province, South Africa. S Afr J Sci. 2010;106(11):1–6. doi:10.4102/sajs.v106i11/12.243
39. Bekele T, Zewde G, Tefera G, Feleke A, Zerom K. Escherichia coli O157: H7 in raw meat in Addis Ababa, Ethiopia: prevalence at an abattoir and retailers and antimicrobial susceptibility. Int J Food Contamin. 2014;1(1):1–8. doi:10.1186/s40550-014-0004-9
40. Shecho M, Thomas N, Kemal J, Muktar Y. Cloacael carriage and multidrug resistance Escherichia coli O157: H7 from Poultry Farms, Eastern Ethiopia. J Vet Med. 2017;2017:1–9. doi:10.1155/2017/8264583
41. Ahmed M, Van Velkinburgh J. Enterohemorrhagic Escherichia coli O157 in North Africa region: a threat requires advanced investigation. Pan Afr Medl J. 2014;19(1). doi:10.11604/pamj.2014.19.26.4825
42. Hiko A, Asrat D, Zewde G. Occurrence of Escherichia coli O157: H7 in retail raw meat products in Ethiopia. J Infect Dev Ctries. 2008;2(05):389–393. doi:10.3855/jidc.203
43. Addis Z, Kebede N, Sisay Z, Alemayehu H, Wubetie A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect Dis. 2011;11(1):1–7. doi:10.1186/1471-2334-11-222
44. Magwira CA, Gashe BA, Collison EK. Prevalence and antibiotic resistance profiles of Escherichia coli O157: H7 in beef products from retail outlets in Gaborone, Botswana. J Food Prot. 2005;68(2):403–406. doi:10.4315/0362-028X-68.2.403
45. Lakshmi V, Ashok R, Susmita J, Shailaja VV. Changing trends in the antibiograms of Salmonella isolates at a tertiary care hospital in Hyderabad. Indian J Med Microbiol. 2006;24(1):45–48. doi:10.1016/S0255-0857(21)02470-1
46. Tesfaw L, Taye B, Alemu S, Alemayehu H, Sisay Z, Negussie H. Prevalence and antimicrobial resistance profile of Salmonella isolates from dairy products in Addis Ababa, Ethiopia. Afr J Microbiol Res. 2013;7(43):5046–5050. doi:10.5897/AJMR2013.5635
47. Tafa F, Terefe Y, Tamerat N, Zewdu E. Isolation, identifications and antimicrobial susceptibility pattern of coagulase positive Staphylococcus from subclinical mastitic dairy cattle in and around Haramaya University. Ethiop Vet J. 2015;19(2):41–53. doi:10.4314/evj.v19i2.8
48. Abera M, Demie B, Aragaw K, Regassa F, Regassa A. Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J Vet Med Animal Health. 2010;2(3):29–34.
49. Mekuria A, Asrat D, Woldeamanuel Y, Tefera G. Identification and antimicrobial susceptibility of Staphylococcus aureus isolated from milk samples of dairy cows and nasal swabs of farm workers in selected dairy farms around Addis Ababa, Ethiopia. Afr J Microbiol Res. 2013;7(27):3501–3510.
50. Santos L, Viana C, Farinha G, Otutumi L, Gerbasi A. Antimicrobial susceptibility of strains of Staphylococcus aureus and Staphylococcus coagulase-negative isolated from cows 'milk with mastitis in the West of Paraná, Brazil. Enciclopédia Biosfera. 2013;9(17):1–15.
51. Bedasa S, Shiferaw D, Abraha A, Moges T. Occurrence and antimicrobial susceptibility profile of Escherichia coli O157: H7 from food of animal origin in Bishoftu town, Central Ethiopia. Int J Food Contamin. 2018;5(1):1–8. doi:10.1186/s40550-018-0064-3
52. Iweriebor BC, Iwu CJ, Obi LC, Nwodo UU, Okoh AI. Multiple antibiotic resistances among Shiga toxin producing Escherichia coli O157 in feces of dairy cattle farms in Eastern Cape of South Africa. BMC Microbiol. 2015;15(1):1–9. doi:10.1186/s12866-015-0553-y
53. Dabassa A, Bacha K. The prevalence and antibiogram of Salmonella and Shigella isolated from Abattoir, Jimma town, Southwestern Ethiopia. Conf Jimma Univ. 2011;169:186–190.
54. Tadesse T, Dabassa A. Prevalence and antimicrobial resistance of Salmonella isolated from raw milk samples collected from Kersa district, Jimma Zone, Southwest Ethiopia. J Med Sci. 2012;12(7):224. doi:10.3923/jms.2012.224.228
55. Aliyo A, Seyoum A, Teklemariam Z. Bacteriological quality and antimicrobial susceptibility patterns among raw milk producers and vendors in Gomole district, Borena zone, Southern Ethiopia. Infect Drug Resist. 2022;1:2589–2602. doi:10.2147/IDR.S364578
56. Garbaj AM, Gawella TB, Sherif JA, et al. Occurrence and antibiogram of multidrug-resistant Salmonella enterica isolated from dairy products in Libya. Vet World. 2022;15(5):1185. doi:10.14202/vetworld.2022.1185-1190
57. Tasnim UT, Islam MT. Pathogenic and drug resistant bacteria in raw milk of Jessore city: a potential food safety threat. Bangladesh J Vet Med. 2015;13(1):71–78. doi:10.3329/bjvm.v13i1.23723
58. Regasa S, Mengistu S, Abraha A. Milk Safety Assessment, Isolation, and Antimicrobial Susceptibility Profile of Staphylococcus aureus in Selected Dairy Farms of Mukaturi and Sululta Town, Oromia Region, Ethiopia. Vet Med Int. 2019;2019:1–11. doi:10.1155/2019/3063185
59. Ssajjakambwe P, Bahizi G, Setumba C, et al. Milk hygiene in rural southwestern Uganda: prevalence of mastitis and antimicrobial resistance profiles of bacterial contaminants of milk and milk products. Vet Med Int. 2017;2017:1–6. doi:10.1155/2017/8710758
60. Sharma C, Rokana N, Chandra M, et al. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front Vet Sci. 2018;4:237. doi:10.3389/fvets.2017.00237
61. Al-Harbi H, Ranjbar S, Moore RJ, Alawneh JI. Bacteria isolated from milk of dairy cows with and without clinical mastitis in different regions of Australia and their AMR profiles. Front Vet Sci. 2021;8:743725. doi:10.3389/fvets.2021.743725
62. Tempini PN, Aly SS, Karle BM, Pereira RV. Multidrug residues and antimicrobial resistance patterns in waste milk from dairy farms in Central California. J Dairy Sci. 2018;101(9):8110–8122. doi:10.3168/jds.2018-14398
63. Saini V, McClure JT, Léger D, et al. Antimicrobial resistance profiles of common mastitis pathogens on Canadian dairy farms. J Dairy Sci. 2012;95(8):4319–4332. doi:10.3168/jds.2012-5373
64. Gebeyehu A, Taye M, Abebe R. Isolation, molecular detection and antimicrobial susceptibility profile of Salmonella from raw cow milk collected from dairy farms and households in southern Ethiopia. BMC Microbiol. 2022;22(1):1. doi:10.1186/s12866-022-02504-2
65. Sudarwanto M, Akineden Ö, Odenthal S, Gross M, Usleber E. Extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae in bulk tank milk from dairy farms in Indonesia. Foodborne Pathog Dis. 2015;12(7):585–590. doi:10.1089/fpd.2014.1895
66. Plassard V, Gisbert P, Granier SA, Millemann Y. Surveillance of extended-spectrum β-lactamase-, cephalosporinase-and carbapenemase-producing gram-negative bacteria in raw milk filters and healthy dairy cattle in three farms in Île-de-France, France. Front Vet Sci. 2021;8:633598. doi:10.3389/fvets.2021.633598
67. Odenthal S, Akineden Ö, Usleber E. Extended-spectrum β-lactamase producing Enterobacteriaceae in bulk tank milk from German dairy farms. Int J Food Microbiol. 2016;238:72–78. doi:10.1016/j.ijfoodmicro.2016.08.036
68. Khoshbakht R, Shahed A, Aski HS. Characterization of extended-spectrum Î'-lactamase-producing Escherichia coli strains isolated from dairy products. J Microbiol Biotechnol Food Sci. 2014;3(4):333–336.
69. Badri AM, Ibrahim IT, Mohamed SG, Garbi MI, Kabbashi AS, Arbab MH. Prevalence of extended spectrum beta lactamase (ESBL) producing Escherichia coli, and Klebsiella pneumoniae isolated from raw milk samples in Al Jazirah state, Sudan. Mol Biol. 2017;7(1):201. doi:10.4172/2168-9547.1000201