A Systematic review on Prevalence, Serotypes and Antibiotic ... - Dove Medical Press
Introduction
Salmonellosis continues to be a serious global public health issue, particularly in developing nations.1,2 One-fourth of 550 million diarrheic people worldwide are thought to contract Salmonella each year as a result of eating contaminated food.3 Human typhoid fever is caused by Salmonella Typhi and Salmonella Paratyphi.4 Some Salmonella are specific to animal species although others have wide variety of animals, in addition to humans, such as Salmonella Typhimurium and Salmonella Enteritidis.5
Red meat, carcasses, slaughterhouse equipment, and utensils can all get contaminated by the mesenteric lymph nodes and faeces of sick cattle, swine, sheep, or reservoir animals.6–10 As a result, eating raw or undercooked meat or eggs results in human infection.11 Salmonella infections can also be carried by human and animal faeces, which can contaminate crops.12 In addition, hospitalization has been implicated as a source of invasive non-typhoidal Salmonella.13 Salmonella infections can lead to outbreaks of human salmonellosis and a variety of clinical symptoms, such as mild gastroenteritis, bacteremia, and extra-intestinal localized infections affecting numerous organs.1
One of the present and expanding global risks to public health is Salmonella drug resistance. According to a study from Mexico, non-typhoidal Salmonella had a resistance range of 16.9% to 40.3% to the antibiotics trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, chloramphenicol, and tetracycline.14 Similar to this, the Salmonella serotypes found in food and people in Italy were resistant to tetracycline (48%) and ampicillin (45%),15 and from China, the NTS resistance pattern to ceftriaxone was 37%.16 Additionally, 36,000 and 33,000 cases of salmonella infections resistant to ciprofloxacin and ceftriaxone, respectively, were reported annually from the United States.17 Salmonella serotypes resistant to ceftriaxone have also been reported in Kenya.18
According to the prior data, an article on Salmonella in Ethiopia had been published since 1985,19 and in 1994, Salmonella Newport had been discovered in suspected food poisoning cases among students and food handlers in Gondar, Ethiopia.20 From various regions in Ethiopia, a lot of papers about Salmonella have been published. These researches looked at the frequency of different Salmonella species, serotypes, and medication resistance patterns in people, animals, and vegetables. Numerous Salmonella isolates with high levels of antibiotic resistance have been discovered.21–24
Ethiopia had vast livestock animals in Africa, and living alongside people is prevalent there.25 This may cause the spread of zoonotic illnesses like non-typhoidal salmonella26 as individuals in Ethiopia do not fully comprehend how zoonoses, such as Salmonella are spreading.27 As a result, large number of vulnerable individuals like malnourished children may be exposed to the invasive form of non-typhoidal Salmonella.28,29
Despite the fragmented local studies about Salmonella that have been reported from various regions of Ethiopia, concise information from a systematic review is more helpful for scientific users to identify gaps for additional studies and for policymakers to develop prevention and control strategies based on the scientific information provided. Thus, we sought to conduct a systematic evaluation of the prevalence, serotypes, and antibiotic resistance of Salmonella in people, animals, and their products in Ethiopia from January 1, 2010, to December 30, 2022.
Methods
Literature Search
To find English-language publications concerning Salmonella that had already been published, we used the Google Scholar and PubMed search engines. Keywords like "prevalence", "incidence", "Salmonella", "Salmonella serotypes", "food handlers", "diarrheic patients", "antibiotic susceptibility", "antimicrobial susceptibility", "antibiotic resistance", "antimicrobial resistance", "animals", "humans", and "Ethiopia" were used to search the included studies.
The Inclusion and Exclusion Standards
Three researchers independently conducted the eligible studies using the PRISMA30 checklist to make sure all pertinent data was included. If a study met the requirements listed below, it was qualified for the systematic review: Salmonella resistance and prevalence in animals and humans are mentioned in the purpose, which is also stated in the design, sample size, description of the microbiological procedures, and number of isolates. The study was published in English. Studies were disregarded if they were duplicates, partial papers, or reported Salmonella without an antimicrobial test.
Obtaining and Analyzing Data
From each examined paper, the names of the authors, the publication year, the kind of media, the study setting, the sampling techniques, the study populations, the type of specimens, the sample size, the number of positive isolates, the antimicrobial susceptibility tests, and the list of serotypes (if any) were taken. Using Excel 2013, frequencies and percentages were examined.
Results
Between January 1, 2010, and December 30, 2022, the search engines Google Scholar and PubMed turned up a total of 207 published papers; 100 of those articles were disregarded from additional examination owing to duplication. After reviewing the 107 research titles and abstracts, it was determined that 52 did not meet the requirements for inclusion. Only 55 articles that met the requirements for eligibility were given a second evaluation, and 12 of them were disqualified for various reasons. Figure 1 shows that our systematic review only comprised 43 papers.
 | Figure 1 Flow Diagram for Selection of Eligible Studies. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. Creative Commons.30 |
Characteristics of Studies on Salmonella among Asymptomatic Food Handlers and Diarrhoea Patients
Of the 43 eligible studies that were conducted, 28 were asymptomatic food handlers from the community and diarrhoeic patients from public health facilities. While 13 types of research involved asymptomatic food handlers working in university and community cafeterias, 15 studies involved children and adults with diarrhoea. The only clinical specimens reported in any of the investigations for Salmonella isolation were faeces. The most frequently employed media were Xylose Lysine Deoxycholate, Deoxycholate Citrate Agar, MacConkey Agar, and Salmonella Shigella Agar. According to Table 1, convenience sampling was used in 19 of the investigations, while simple random sampling was used in the other nine.
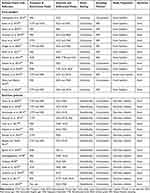 | Table 1 A Systematic Review of Characteristics of Asymptomatic Food Handlers and Public Health Facility Diarrheic Patients in Ethiopia, January 1, 2010, to December 30, 2022 |
Salmonella Prevalence in Asymptomatic Food Handlers and Diarrheal Patients
Salmonella prevalence in asymptomatic food handlers (n=13) ranges from 1% to 10%, but it ranges from 1% to 13% in diarrhoeic patients (n=15). More than three-fourths of the reports among asymptomatic food workers had prevalence rates that were less than 5%. On the other hand, in more than two-thirds of the cases, including diarrheal patients, the prevalence rates were 5% or higher as shown in Table 2.
 | Table 2 A Systematic Review of the Prevalence of Salmonella Among Asymptomatic Food Handlers and Public Health Facilities Diarrheic Patients in Ethiopia from January 1, 2010, to December 30, 2022 |
Characteristics of Research on Salmonella in Animal-Related Samples
The specimens used to isolate Salmonella came from cattle, poultry, pigs, goats, and sheep. These included the contents of the caecum, mesenteric lymph nodes, liver, tongue, hide swabs, rumen, faeces, and cecum, as well as the contents of market eggs and market eggshells. All studies involving animals used the following essential pre-enrichment, enrichment, and selective media: buffered peptone water, brilliant green phenol red lactose sucrose, xylose lysine deoxycholate, MacConkey agar, brilliant green agar, xylose lysine territory 4, selenite cysteine broth, Rappaport-Vassiliadis, and Tetrathionate broth.
Salmonella is present in all of the animal-related specimens, with prevalence ranging from 3.1% in poultry to 29.1% in pig mesenteric lymph nodes. Table 3 lists the results of the investigations that examined the tissue and faeces of pigs, cattle, poultry, sheep, and goats.
 | Table 3 A Systematic Review of the Prevalence of Salmonella in Animals Associated Specimens in Ethiopia from January 1, 2010, to December 30, 2022 |
Salmonella Antibiotic Resistance Among Food Handlers and Diarrheal Patients
A minimum of nine antibiotics were used to assess the resistance of Salmonella isolates. Amoxicillin, ampicillin, tetracycline, trimethoprim-sulfamethoxazole, chloramphenicol, nalidixic acid, gentamicin, ciprofloxacin, and ceftriaxone were on the lists of antibiotics taken from the studies that qualified. In six investigations for amoxicillin (two from food handlers and four from diarrhoea patients) and eight for ampicillin (four from food handlers and four from diarrhoea patients), all of the isolates displayed 100% resistance. Seven investigations (three in food handlers and four in diarrhoea patients) and eight (two in food handlers and six in diarrhoea patients), respectively, identified resistant isolates for ciprofloxacin and ceftriaxone. Although diarrhoeic patients appear to have relatively significant resistance, Table 4 shows that the highest reported ciprofloxacin and ceftriaxone resistance rates were 16.7% and 100%, respectively. Among all the asymptomatic food handlers and diarrheal patients tested for Salmonella, only one study was found eligible for serotyping. The leading serotype that reported from that study was S. Typhimurium, whereas single isolate of S. Concord showed 100% resistance to amoxicillin, ampicillin, tetracycline, gentamicin, sulfamethoxazole trimethoprim and ciprofloxacin as indicated in Table 4.
 | Table 4 A Systematic Review of Antibiotic Resistance of Salmonella Among Asymptomatic Food Handlers and Public Health Facility Diarrheic Patients in Ethiopia from January 1, 2010 to December 30, 2022 |
Animal Salmonella with Antibiotic Resistance
As shown in Table 5, all Salmonella isolates from investigations involving animal-related samples were resistant to ampicillin, 90% to streptomycin, 66.7% to chloramphenicol, 86.4% to tetracycline, 35.2% to ciprofloxacin, 29.4% to gentamicin, and 23.2% to ceftriaxone. When it comes to salmonella serotypes' resistance to antibiotics, the ampicillin, gentamicin, ciprofloxacin, streptomycin, and tetracycline resistance in S. Kentucky was 100% although the leading isolates of salmonella serotypes reported from the researches were S. Anatum, S. Saintpaul, S. Newport, and S. Typhimurium.
 | Table 5 A Systematic Review of Antibiotic Resistance of Salmonella in Species and Serotype Level in Animals Related Specimens in Ethiopia from January 1, 2010 to December 30, 2022 |
Discussion
One of the main contributors to foodborne and zoonotic diseases in underdeveloped nations is Salmonella.1,2 It is one of Ethiopia's top seven priority zoonotic diseases73 and a significant source of foodborne pathogens.74
Salmonella prevalence among asymptomatic food handlers ranges from 1% to 10%, which was consistent with research from Pakistan (9.1%).75 Similar to this, the prevalence of Salmonella among individuals who have diarrhoea ranges from 1% to 13%. However, one instance at a hospital suggests that there are 30 other cases in the community who are unable to visit hospitals for a variety of reasons.76
The prevalence rates of Salmonella in animal-related sources range from 3.1% in poultry to 29.4% in pigs, followed by cloacal and cecal content of chicken (24.6%) which is consistent with studies from Burkina Faso,77,78 Italy,79 Kenya,80 South Africa,81 and Uganda,82 Vietnam,83 China,84 and Louisiana.85
S. Typhimurium were reported in all of the legible studies for serotyping which was also reported from Gambia,86 Mali,87 India,88 Mexico,89 and China.90 According to reports from sub-Saharan African nations, S. Typhimurium and S. Enteritidis are invasive forms of NTS, especially among susceptible people, such as those with HIV, malnourished children, and in malaria areas.77,91 It is possible that the existence of such Salmonella germs in cattle, poultry, pigs, and other animal sources is regarded as a potential source of contamination in humans and maybe the main risk factor for Salmonella outbreaks in humans.8
Six human studies found that every isolate of Salmonella tested had a 100% amoxicillin resistance rate, which is consistent with an Ethiopian study.24 Ciprofloxacin-resistant isolates were detected in seven human studies which is similar to studies from China,92 and Mexico.93
Salmonella isolates from two investigations had 100% ampicillin resistance, which was higher than the prior study in Japan.6 However, the streptomycin resistance patterns were consistent with earlier Japanese studies.6 For ciprofloxacin, gentamicin, and ceftriaxone, similar animal specimens showed at least 25% resistance. S. Kentucky showed 100% resistance to ampicillin, gentamicin, ciprofloxacin, streptomycin, and tetracycline out of the known serotypes from our systematic evaluation.
The results of our systematic review are very beneficial in helping people understand important information concerning Salmonella. We were unable to include Salmonella in food, fruits, or vegetables because of limited data. Additionally, no studies that were included in this systematic review performed molecular characterization of Salmonella and its resistance genes or invasive non-typhoidal salmonella among malnourished children because of no studies related.
Conclusions
The prevalence of Salmonella in animals, diarrhoeal patients, and asymptomatic food handlers was high. The prevalence of Salmonella among asymptomatic food handlers and diarrheal patients was nearly similar. S. Typhimurium that has the zoonotic nature was recovered from human and animal studies. Studies on animals revealed high levels of resistance to tetracycline, ampicillin, and streptomycin. Circulating of Salmonella in the community in Ethiopia is a homework for academic and non-academic researchers to overcome fear of future outbreaks. National and international organizations should work on strengthening the prevention and control of salmonellosis. Due to Ethiopia's high prevalence of underweight children and the prevalence of those who are susceptible to the invasive form of NTS, invasive non-typhoidal salmonella among malnourished children should be examined.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lo HY, Lai FP, Yang YJ. Changes in epidemiology and antimicrobial susceptibility of nontyphoidal Salmonella in children in southern Taiwan, 1997–2016. J Microbiol Immunol Infect. 2020;53(4):585–591. doi:10.1016/j.jmii.2018.06.004
2. Stanaway J, Parisi A, Sarkar K, et al. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(12):1312–1324. doi:10.1016/S1473-3099(19)30418-9
3. World Health Organization. Salmonella (non-typhoidal); 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/salmonella.
4. Ikhimiukor O, Oaikhena A, Afolayan AO, et al. Genomic characterization of invasive typhoidal and non-typhoidal Salmonella in southwestern Nigeria. Genomic characterization of invasive typhoidal and non-typhoidal Salmonella in southwestern Nigeria. PLoS Negl Trop Dis. 2022;16(8):e0010716. doi:10.1371/journal.pntd.0010716
5. Hawwas HA, Aboueisha AM, Fadel HM, ElMahallawy HS. Salmonella serovars in sheep and goats and their probable zoonotic potential to humans in Suez Canal Area. Egypt Cta Veterinaria Scandinavica. 2022;64(1):17. doi:10.1186/s13028-022-00637-y
6. Duc VM, Nakamoto Y, Fujiwara A, et al. Prevalence of Salmonella in broiler chickens in Kagoshima, Japan in 2009 to 2012 and the relationship between serovars changing and antimicrobial resistance. BMC Vet Res. 2019;15(1):108. doi:10.1186/s12917-019-1836-6
7. Mannion C, June F, McLernon J, et al. The role of transport, lairage and slaughter processes in the dissemination of Salmonella spp. in pigs in Ireland. Food Res Int. 2012;45(2):871–879. doi:10.1016/j.foodres.2011.02.001
8. Hugas M, Beloeil PA. Controlling Salmonella along the food chain in the European Union - progress over the last ten years. Euro Surveill. 2014;19(19):20804. doi:10.2807/1560-7917.ES2014.19.19.20804
9. Boschi-Pinto C, Young M, Black RE. The Child Health Epidemiology Reference Group reviews the effectiveness of interventions to reduce maternal, neonatal and child mortality. Int J Epidemiol. 2010;39(Supplement 1):3–6. doi:10.1093/ije/dyq018
10. Reza HS, Zohreh M, Amir G. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from retail chicken meat and giblets in Iran. J Infect Dev Countr. 2015;9(05):463–469. doi:10.3855/jidc.5945
11. Jacob ST, Moore CC, Banura P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One. 2009;4(11):7782. doi:10.1371/journal.pone.0007782
12. Kowalska B. Fresh vegetables and fruit as a source of Salmonella bacteria. Ann Agric Environ Med. 2023;30(1):9–14. doi:10.26444/aaem/156765
13. Oxman JM, Boadla LR, Sells N. An isolated case of nosocomial acquisition of invasive non-typhoidal Salmonella. IDCases. 2023;33:01816. doi:10.1016/j.idcr.2023.e01816
14. Delgado-Suarez EJ, Guiterrez TP, Ruıza-Lo´pez FA, et al. Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant nontyphoidal Salmonella in Mexico. PLoS One. 2021;16(5):e0243681. doi:10.1371/journal.pone.0243681
15. Capuano F, Mancusi A, Capparelli R, et al. Characterization of Drug Resistance and Virulotypes of Salmonella Strains Isolated from Food and Humans. Foodborne Pathog Dis. 2013;10(11):963–968. doi:10.1089/fpd.2013.1511
16. Ke Y, Lu W, Liu W, et al. Non-typhoidal Salmonella infections among children in a tertiary hospital in Ningbo, Zhejiang, China, 2012–2019. PLoS Negl Trop Dis. 2020;14(10):e0008732. doi:10.1371/journal.pntd.0008732
17. Centers for Disease Control and Prevention. Antibiotic Resistance Threats In the United States; 2013.
18. Mutai WC, Muigai AW, Waikiki P, Kariuki S. Multi-drug resistant Salmonella enterica serovars Typhi isolates with reduced susceptibility to ciprofloxacin in Kenya. BMC Microbiol. 2018;18(1):187. doi:10.1186/s12866-018-1332-3
19. Gebre-Yohannes A. Salmonella from Ethiopia: prevalent species and their susceptibility to drugs. Ethiopia Med J. 1985;23(3):97–102.
20. Aseffa A, Mengistu G, Tiruneh M. Salmonella Newport: outbreak of food poisoning among college students due to contaminated undercooked eggs. Ethiopia Med J. 1994;32:1–6.
21. Guchi B, Ashenafi M. Microbial Load, Prevalence and Antibiograms of Salmonella and Shigella in Lettuce and Green Peppers. Ethiop J Health Sci. 2010;20:42–48.
22. Eguale T, Engidawork E, Gebreyes WA, et al. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC Microbiol. 2016;16(1):20. doi:10.1186/s12866-016-0638-2
23. Mitiku H, Weldegebreal F, Marami D, Teklemariam Z. Nontyphoidal Salmonella bacteremia in antiretroviral therapy-naïve HIV-infected individuals at three public hospitals in eastern Ethiopia: prevalence, antimicrobial susceptibility patterns, and associated factors. HIV/AIDS. 2019;2019:11 23–29.
24. Dessale M, Mengistu G, Mengist HM. Prevalence, antimicrobial resistance pattern, and associated factors of Salmonella and Shigella among under five diarrheic children attending public health facilities in Debre Markos town, Northwest Ethiopia. Front Public Health. 2023;11. doi:10.3389/fpubh.2023.1114223
25. Food and Agriculture Organization of the United Nations. The Future of Livestock in Ethiopia. Opportunities and Challenges in the Face of Uncertainty. Rome: Food and Agriculture Organization of the United Nations; 2019.
26. Hoelzer K, Andrea Switt AIM, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34. doi:10.1186/1297-9716-42-34
27. Alebie A, Tewachew T. Household practice related to zoonotic diseases transmission in rural community of Gondar Zuria District. Vet Med. 2021;12:109–115.
28. Mtove G, Amos B, von Seidlein L, et al. Invasive salmonellosis among children admitted to a rural Tanzania hospital and a comparison with previous studies. PLoS One. 2010;5(2):e9244. doi:10.1371/journal.pone.0009244
29. Abebe HF, Taffere GR, Fisseha MA, Bezabih AM. Risk factors and spatial distributions of underweight among children under-five in urban and rural communities in Tigray, Northern Ethiopia: using ordinal logistic regression analysis. Nutr Dietary Suppl. 2022;14:21–37. doi:10.2147/NDS.S371773
30. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
31. Gebreyesus A, Adane K, Negash L, et al. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Mekelle University student cafeteria, Mekelle, Ethiopia. Food Control. 2014;44:45–48. doi:10.1016/j.foodcont.2014.03.040
32. Getie M, Abebe W, Tessema B. Prevalence of enteric bacteria and their antimicrobial susceptibility patterns among food handlers in Gondar town, Northwest Ethiopia. Antimicrob Resist Infect Control. 2019;8(1):111. doi:10.1186/s13756-019-0566-7
33. Abera B, Biadegelgen F, Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiop J Health Dev. 2010;24:46–51.
34. Solomon F, Wada FW, Anjulo AA, et al. Burden of intestinal pathogens and associated factors among asymptomatic food handlers in South Ethiopia: emphasis on salmonellosis. BMC Res Notes. 2018;11(1):502. doi:10.1186/s13104-018-3610-4
35. Awol N, Nigusse D, Ali M. Prevalence and antimicrobial susceptibility profile of Salmonella and Shigella among food handlers working in food establishment at Hawassa city, Southern Ethiopia. BMC Res Notes. 2019;12(1):712. doi:10.1186/s13104-019-4725-y
36. Yesigat T, Jemal M, Birhan W. Prevalence and associated risk factors of Salmonella, Shigella, and intestinal parasites among food handlers in Motta Town, North West Ethiopia. Can J Infect Dis Med Microbiol. 2020;2020:1–11. doi:10.1155/2020/6425946
37. Aklilu AK, Dessalegn M, Tarekegn N, et al. Prevalence of intestinal parasites, Salmonella and Shigella among apparently healthy food handlers of Addis Ababa University student's cafeteria, Addis Ababa, Ethiopia. BMC Res Notes. 2015;8(1):17. doi:10.1186/s13104-014-0967-x
38. Kifelew GL, Wondafrash N, Feleke A. Identification of drug-resistant Salmonella from food handlers at the University of Gondar, Ethiopia. BMC Res Notes. 2014;7(1):545. doi:10.1186/1756-0500-7-545
39. Getnet F, Gebre-Selassie S, Alemayehu H, et al. Prevalence and antimicrobial resistance of salmonella isolated from food handlers in Addis Ababa University Students' Cafeteria, Ethiopia. Afr J Basic Appl Sci. 2014;6(6):210–216.
40. Mengist A, Mengistu G, Reta A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. Int J Infect Dis. 2018;75:74–79. doi:10.1016/j.ijid.2018.08.008
41. Tadesse G, Mitiku H, Teklemariam Z, Marami D. Salmonella and shigella among asymptomatic street food vendors in the Dire Dawa city, Eastern Ethiopia: prevalence, antimicrobial susceptibility pattern, and associated factors. Environ Health Insights. 2019;13:1–8. doi:10.1177/1178630219853581
42. Mama M, Alemu G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect Dis. 2016;16(1):686. doi:10.1186/s12879-016-2035-8
43. Diriba K, Awulachew E, Ashuro Z. Prevalence and antimicrobial resistance pattern of Salmonella, Shigella, and intestinal parasites and associated factor among Food Handlers in Dilla University Student Cafeteria, Dilla, Ethiopia. Int J Microbiol Res. 2020;2020:1–10. doi:10.1155/2020/3150539
44. Tosisa W, Mihret A, Ararsa A, et al. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Pediatr. 2020;20(91). doi:10.1186/s12887-020-1970-0
45. Abebe W, Earsido A, Taye S, et al. Prevalence and antibiotic susceptibility patterns of Shigella and Salmonella among children aged below five years with Diarrhoea attending Nigist Eleni Mohammed Memorial Hospital, South Ethiopia. BMC Pediatr. 2018;18(1):241. doi:10.1186/s12887-018-1221-9
46. Getamesay M, Getent B, Ahmed Z. Prevalence of Shigella, Salmonella and Campylobacter species and their susceptibility patterns among under-five children with diarrhoea in Hawassa town, south Ethiopia. Ethiop J Health Sci. 2014;24(2):101–108. doi:10.4314/ejhs.v24i2.1
47. Mamuye Y, Metaferia G, Birhanu A, et al. Isolation and antibiotic susceptibility patterns of Shigella and Salmonella among under 5 children with acute diarrhoea: a cross-sectional study at selected public health facilities in Addis Ababa, Ethiopia. Clin Microbiol. 2015;4(01). doi:10.4172/2327-5073.1000186
48. Mulu W, Abera B, Yimer M, et al. Bacterial agents and antibiotic resistance profiles of infections from different sites that occurred among patients at Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10(1):254. doi:10.1186/s13104-017-2584-y
49. Kebede A, Solomon A, Tchalew TS. The common enteric bacterial pathogens and their antimicrobial susceptibility patterns among HIV-infected individuals attending the antiretroviral therapy clinic of Hawassa University Hospital, southern Ethiopia. Antimicrob Resist Infect Control. 2017;6(128). doi:10.1186/s13756-017-0288-7
50. Amsalu TG, Adem Siraj Y, Adem Siraj Y. Salmonella Typhi and Salmonella Paratyphi prevalence, antimicrobial susceptibility profile and factors associated with enteric fever infection in Bahir Dar, Ethiopia. Sci Rep. 2021;11(1):7359. doi:10.1038/s41598-021-86743-9
51. Beyene G, Tasew H. Prevalence of intestinal parasite, Shigella and Salmonella species among diarrheal children in Jimma health centre, Jimma southwest Ethiopia: a cross-sectional study. Ann Clin Microbial Antimicrobial. 2014;13(10):1–7.
52. Eguale T, Gebreyes WA, Asrat D, et al. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhoea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis. 2015;15(1):497. doi:10.1186/s12879-015-1235-y
53. Gebreegziabher G, Asrat D, W/Amanuel Y, Hagos T. Isolation and antimicrobial susceptibility profile of Shigella and Salmonella Species from children with acute diarrhoea in Mekelle Hospital and Semen Health Center, Ethiopia. Ethiopia J Health Sci. 2018;28(2):197–206.
54. Terfassa A, Jida M. Prevalence and Antibiotics Susceptibility Pattern of Salmonella and Shigella Species among Diarrheal Patients Attending Nekemte Referral Hospital, Oromia, Ethiopia. Int J Microbiol. 2018;2018:9214689. doi:10.1155/2018/9214689
55. Assefa A, Girma M. Prevalence and antimicrobial susceptibility patterns of Salmonella and Shigella isolates among children aged below five years with diarrhoea attending Robe General Hospital and Goba Referral Hospital, South East Ethiopia. Trop Dis Travel Med Vaccines. 2019;5:19. doi:10.1186/s40794-019-0096-6
56. Lamboro T, Ketema T, Bacha K. Prevalence and antimicrobial resistance in Salmonella and Shigella species isolated from outpatients, Jimma University Specialized Hospital, Southwest Ethiopia. Can J Infect Dis Med Microb. 2016;2016:4210760.
57. Reda A, Seyoum B, Andualem YG, et al. Antibiotic susceptibility patterns of Salmonella and Shigella isolates in Harar, Eastern Ethiopia. J Infect Dis Immun. 2011;3(8):134–139.
58. Ameya G, Tsalla T, Getu F, Getu E. Antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella infections among under-five children in Arba Minch, South Ethiopia. Ann Clin Microbiol Antimicrob. 2018;17(1):1. doi:10.1186/s12941-018-0253-1
59. Dagnew B, Alemayehu H, Medhin G, Eguale T. Prevalence and antimicrobial susceptibility of Salmonella in poultry farms and in-contact humans in Adama and Modjo towns, Ethiopia. Microbiol Open. 2020;9(8):e1067. doi:10.1002/mbo3.1067
60. Worku WD, Menjetta T, Menjetta T. High prevalence and antimicrobial susceptibility pattern of salmonella species and extended-spectrum beta-lactamase producing Escherichia coli from raw cattle meat at butcher houses in Hawassa city, Sidama regional state, Ethiopia. PLoS One. 2022;17(1):e0262308. doi:10.1371/journal.pone.0262308
61. Taddese D, Tolosa T, Deresa B, et al. Antibiograms and risk factors of Salmonella isolates from laying hens and eggs in Jimma Town, South Western Ethiopia. BMC Res Notes. 2019;12(1):472. doi:10.1186/s13104-019-4516-5
62. Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: prevalence and antimicrobial resistance. BMC Vet Res. 2018;14(1):217. doi:10.1186/s12917-018-1539-4
63. Alemu S, Zewde BM. Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Trop Anim Health Prod. 2012;44(3):595–600. doi:10.1007/s11250-011-9941-y
64. Mustefa BA, Gebremedhin EZ. Carriage and antimicrobial resistance of non-typhoidal Salmonella in cattle slaughtered in Ambo municipality abattoir, West Shewa zone, Oromia, Ethiopia - a point prevalence survey. Ethiop Vet J. 2018;22(2):94. doi:10.4314/evj.v22i2.8
65. Alemu A, Regassa F, Kebede N, et al. The magnitude and Antimicrobial Susceptibility Profile of Salmonella Recovered from Export Abattoirs Located in East Shewa, Ethiopia. Infect Drug Resist. 2022;15:1353–1365. doi:10.2147/IDR.S348773
66. Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Popul Nutr. 2017;36(1):52. doi:10.1186/s41043-017-0131-z
67. Addis Z, Kebede N, Sisay Z, et al. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross-sectional study. BMC Infect Dis. 2011;11(1):222. doi:10.1186/1471-2334-11-222
68. Takele S, Woldemichael K, Gashaw M, et al. Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at the Jimma municipal abattoir, southwest Ethiopia. Trop Dis Travel Med Vaccines. 2018;4(1):13. doi:10.1186/s40794-018-0072-6
69. Mohammed Y, Dubie T. Isolation, identification and antimicrobial susceptibility profile of Salmonella isolated from poultry farms in Addis Ababa, Ethiopia. Vet Med Sci. 2022;8(3):1166–1173. doi:10.1002/vms3.762
70. Sibhat B, Zewde BM, Zerihun A, et al. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health. 2011;58(2):102–109. doi:10.1111/j.1863-2378.2009.01305.x
71. Usmael B, Abraha B, Alemu S, et al. Isolation, antimicrobial susceptibility patterns, and risk factors assessment of nontyphoidal Salmonella from apparently healthy and diarrheic dogs. BMC Vet Res. 2022;18(1):37. doi:10.1186/s12917-021-03135-x
72. Belachew TM, Roba E, Asefa YT, et al. Prevalence and Antimicrobial-Susceptibility Profiles of Salmonella in Smallhold Broiler Supply Chains in Central Ethiopia. Infect Drug Resist. 2021;14:4047–4055. doi:10.2147/IDR.S331249
73. Centers for Disease Control and Prevention, Ethiopia Public Health Institute and Ethiopian Ministry of Agriculture. Zoonotic Disease Prioritization for Inter-sectoral Engagement in Ethiopia. Addis Ababa; 2015.
74. Asfaw T, Genetu D, Shenkute D, et al. Foodborne pathogens and antimicrobial resistance in Ethiopia: an urgent call for action on "One Health". Infect Drug Resist. 2022;15:5265–5274. doi:10.2147/IDR.S375043
75. Siddiqui TR, Bibi S, Mustufa MA, et al. High prevalence of typhoidal Salmonella enterica serovars excreting food handlers in Karachi-Pakistan: a probable factor for regional typhoid endemicity. J Health Popul Nutr. 2015;33(1):27. doi:10.1186/s41043-015-0037-6
76. Centers for Diseases Control and Prevention. Food safety: Salmonella and food. Available from: https://www.cdc.gov/foodsafety/communication/salmonella-food.html.
77. Post AS, Diallo SN, Guiraud I, et al. Supporting evidence for a human reservoir of invasive non Typhoidal Salmonella from household samples in Burkina Faso. PLoS Negl Trop Dis. 2019;13(10):e0007782. doi:10.1371/journal.pntd.00077
78. Kagambèga A, Lienemann T, Aulu L, et al. Prevalence and characterization of Salmonella enterica from the faeces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbiol. 2013;13(1):253. doi:10.1186/1471-2180-13-253
79. Busani L, Cigliano A, Taioli E, et al. Prevalence of Salmonella enterica and Listeria monocytogenes contamination in foods of animal origin in Italy. J Food Prot. 2005;68(8):1729–1733. doi:10.4315/0362-028X-68.8.1729
80. Langata LM, Maingi JM, Musonye HA, et al. Antimicrobial resistance genes in Salmonella and Escherichia coli isolates from chicken droppings in Nairobi, Kenya. BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-019-4068-8
81. Magwedere K, Rauff D, De Klerk G, et al. Incidence of nontyphoidal Salmonella in food-producing animals, animal feed, and the Associated Environment in South Africa, 2012–2014. Clin Infect Dis. 2015;61(suppl 4):283–289. doi:10.1093/cid/civ663
82. Odoch T, Wasteson Y, Abée-Lund T, et al. Prevalence, antimicrobial susceptibility and risk factors associated with non-typhoidal Salmonella on Ugandan layer hen farms. BMC Vet Res. 2017;13(1):365. doi:10.1186/s12917-017-1291-1
83. Quynh HL, Fries R, Padungtod PP, et al. Prevalence of Salmonella in Retail Chicken Meat in Hanoi, Vietnam. Ann N Y Acad Sci. 2006;1081(1):257–261. doi:10.1196/annals.1373.032
84. Baowei Y, Meili X, Xin W, et al. Prevalence of Salmonella on raw poultry at retail markets in China. J Food Prot. 2011;74(10):1724–1728. doi:10.4315/0362-028X.JFP-11-215
85. Lestari SI, Feifei H, Wang FEI, Beilei G. Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. J Food Prot. 2009;72(6):1165–1172. doi:10.4315/0362-028X-72.6.1165
86. Dione M, Ikumapayi UN, Saha D, et al. Clonal Differences between Non-Typhoidal Salmonella (NTS) Recovered from Children and Animals Living in Close Contact in The Gambia. PLoS Negl Trop Dis. 2011;5(5):e1148. doi:10.1371/journal.pntd.0001148
87. Sharon M, Tennant SD, Levy H, et al. Identification by PCR of Non-typhoidal Salmonella enterica Serovars Associated with Invasive Infections among Febrile Patients in Mali. PLoS Negl Trop Dis. 2010;4(3):e621.
88. Saravanan S, Purushothaman V, Murthy TR, et al. Molecular epidemiology of Nontyphoidal Salmonella in poultry and poultry products in India: implications for human health. Indian J Microbiol. 2015;55(3):319–326. doi:10.1007/s12088-015-0530-z
89. Quiroz-Santiago C, Rodas-Suárez OR, Vázquez QCR, et al. Prevalence of Salmonella in vegetables from Mexico. J Food Prot. 2009;72(6):1279–1282. doi:10.4315/0362-028X-72.6.1279
90. Liang Z, Ke B, Deng X, et al. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect Dis. 2015;15(1):53. doi:10.1186/s12879-015-0784-4
91. Oneko M, Kariuki S, Muturi-Kioi V, et al. Emergence of community-acquired, multidrug-resistant invasive nontyphoidal Salmonella Disease in Rural Western Kenya, 2009–2013. Clin Infect Dis. 2015;61(suppl 4):310–316. doi:10.1093/cid/civ674
92. Gao F, Huang Z, Xiong Z, et al. Prevalence, serotype, and antimicrobial resistance profiles of children infected with Salmonella in Guangzhou, southern China, 2016–2021. Front Pediatric. 2023;11:1077158. doi:10.3389/fped.2023.1077158
93. Delgado-Sua´rez EJ, Palo´s-Guite´rrez T, Ruı´za Lo´pez FA, et al. Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant nontyphoidal Salmonella in Mexico. PLoS One. 2020;16(5):e0243681. doi:10.1371/journal.pone.0243681
Comments
Post a Comment