Testing Algorithm for Histoplasmosis | Histoplasmosis - CDC
When to consider testing for histplasmosis
PDF version
Overview
Histoplasmosis is a fungal infection that often presents as community-acquired pneumonia (CAP) in primary and urgent care settings. It is endemic in parts of the U.S. and the world.
Histoplasmosis cannot be reliably distinguished from other causes of respiratory illness by signs or symptoms alone. Thus, patients are commonly misdiagnosed; in some instances, over half of patients may receive inappropriate antibacterial drugs.1
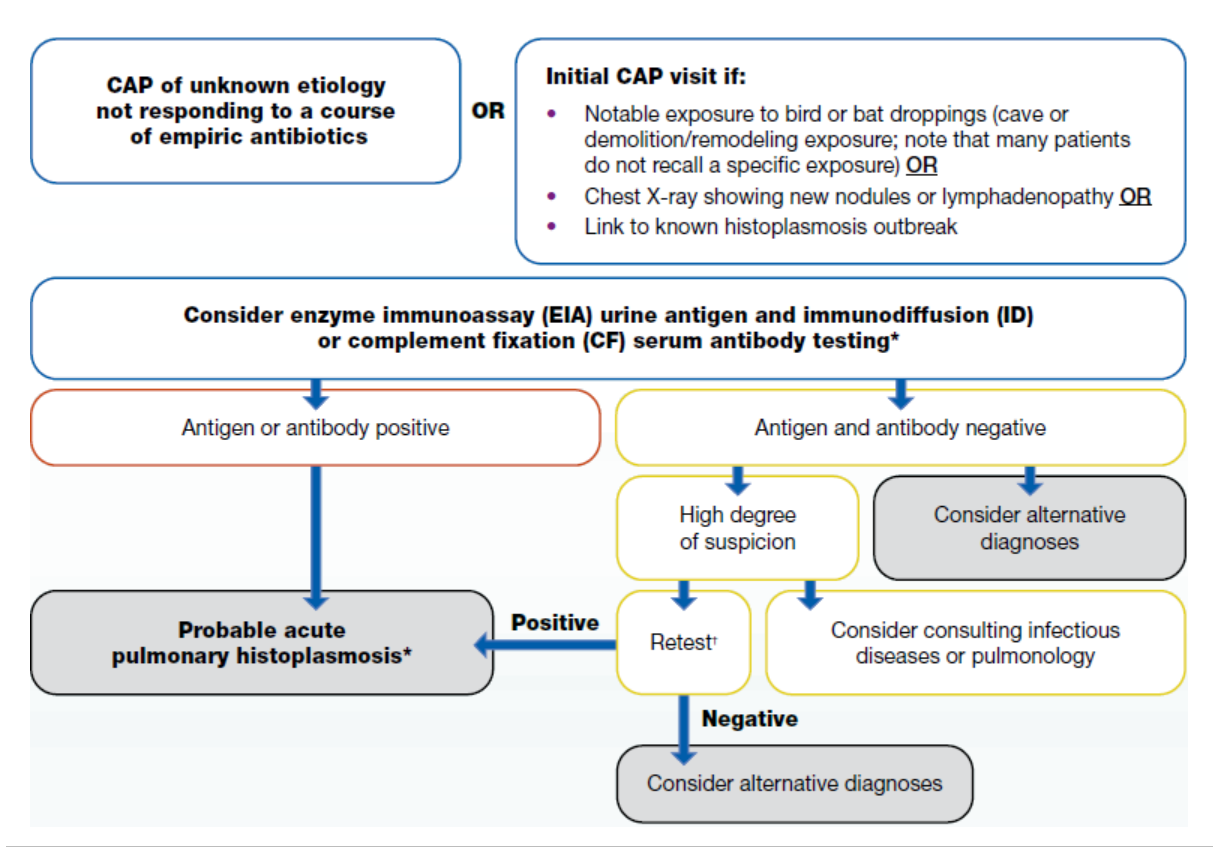
Consider testing patients with community-acquired pneumonia who:
- Live in or recently traveled to an endemic area (maps below) and
- Have not improved with at least one course of empiric antibiotics.
Visual algorithm
If a patient lives in or has traveled to a histoplasmosis-endemic area and has:
Approximate histoplasmosis-endemic areas
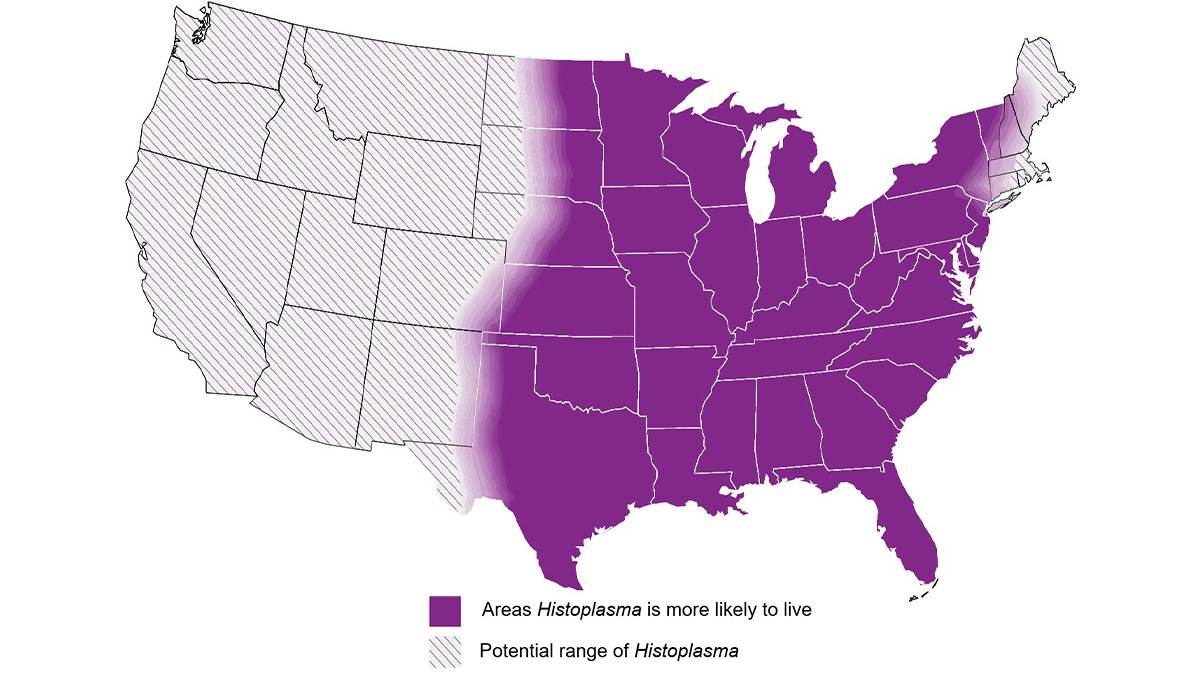
These areas include central and eastern United States, especially around the Ohio and Mississippi River Valleys
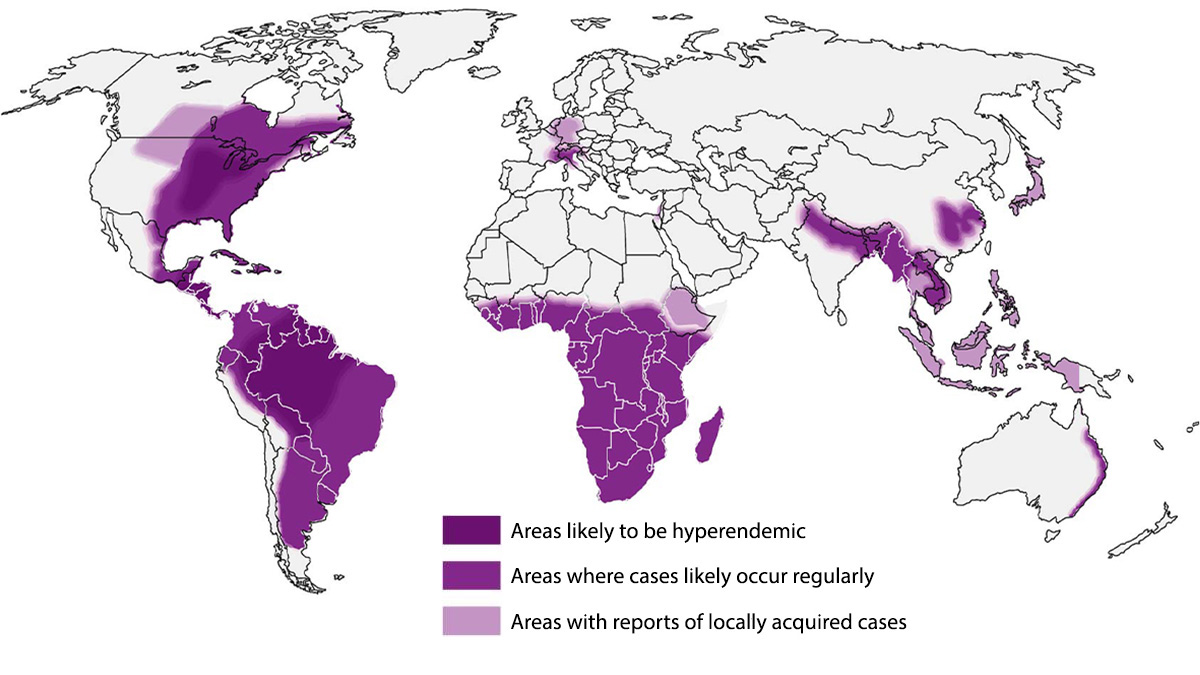
Globally, Histoplasmosis is endemic in parts of South and Central America, Africa, Asia and Europe.
Additional considerations
Clinicians may also consider testing for histoplasmosis on an initial presentation of community-acquired pneumonia when patients have any of the following features:
- Notable exposure to bird or bat droppings (e.g., entered cave, demolition/remediation of building with extensive droppings); be aware most patients do not recall such exposures
- Chest X-ray demonstrating new nodules or lymphadenopathy
- Link to known histoplasmosis outbreak
Disease can occur in both immunocompetent and immunocompromised persons. Travel-associated disease is common, and climate change may be influencing the geographical range of Histoplasma; thus, cases can be found in all regions across the United States.
How to test for histoplasmosis
We recommend ordering enzyme immunoassay (EIA) urine antigen tests. Clinicians may consider obtaining a serum specimen to test concurrently for antibody by immunodiffusion (ID) or complement fixation (CF) which may increase sensitivity of diagnosis; false positives from previous infection can occur, but antibody positivity typically wanes within three years after infection. If these tests are negative, clinicians should consider alternative diagnoses. If a high degree of suspicion remains despite negative initial testing, a clinician may consider ordering (or repeating) antibody testing since tests may be negative early in illness. At this point, clinicians might also consider ordering a sputum or bronchoalveolar lavage (BAL) culture and microscopy. Consultation with specialists in infectious diseases or pulmonology may be considered when a high degree of suspicion remains as well.
Histoplasma antigen and antibody tests have extensive cross-reactivity with Blastomyces. Both infections are treated the same way in most forms.
Clinicians should refer to the Infectious Diseases Society of America's histoplasmosis treatment guidelines or an infectious disease physician when determining therapy after a positive result.
| Test | Sensitivity | Specificity | Population Studied |
|---|---|---|---|
| Antibody Tests | |||
| EIA antibody | 98% | 97% (high cross-reactivity with Blastomyces) | Immunocompromised & healthy populations |
| Complement fixation (CF) antibody | 72%–95% | 70%–80% | Adult populations |
| Immunodiffusion (ID) antibody | 70%–95% | 100% | Adult populations |
| Antigen tests | |||
| EIA Urine antigen | 79% | 99% | Adult population, people living with HIV |
| EIA Serum antigen | 82% | 97% | Adult population, people living with HIV |
| Other tests | |||
| Culture | 15%–85% | 100% | Acute or subacute, disseminated disease |
| Microscopy/histopathology | 9%–43% | 100% | Acute or subacute, disseminated disease |
Comments
Post a Comment