Evidence of Helicobacter pylori heterogeneity in human stomachs by susceptibility testing and characterization of ... - Nature.com
Abstract
Heterogeneity of Helicobacter pylori communities contributes to its pathogenicity and diverse clinical outcomes. We conducted drug-susceptibility tests using four antibiotics, clarithromycin (CLR), amoxicillin (AMX), metronidazole and sitafloxacin, to examine H. pylori population diversity. We also analyzed genes associated with resistance to CLR and AMX. We examined multiple isolates from 42 Japanese patients, including 28 patients in whom primary eradication with CLR and AMX had failed, and 14 treatment-naïve patients. We identified some patients with coexistence of drug resistant- and sensitive-isolates (drug-heteroR/S-patients). More than 60% of patients were drug-heteroR/S to all four drugs, indicating extensive heterogeneity. For the four drugs except AMX, the rates of drug-heteroR/S-patients were higher in treatment-naïve patients than in primary eradication-failure patients. In primary eradication-failure patients, isolates multi-resistant to all four drugs existed among other isolates. In primary eradication-failure drug-heteroR/S-patients, CLR- and AMX-resistant isolates were preferentially distributed to the corpus and antrum with different minimum inhibitory concentrations, respectively. We found two mutations in PBP1A, G591K and A480V, and analyzed these in recombinants to directly demonstrate their association with AMX resistance. Assessment of multiple isolates from different stomach regions will improve accurate assessment of H. pylori colonization status in the stomach.
Similar content being viewed by others
Helicobacter pylori infection and antibiotic resistance — from biology to clinical implications

Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years

Analysis of Helicobacter pylori resistance in patients with different gastric diseases
Introduction
Helicobacter pylori is a highly genetically diverse, gram-negative, microaerophilic pathogenic bacterium that colonizes the stomach1,2. H. pylori is thought to cause gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric lymphoma3,4,5. H. pylori is also involved in the development of extra-gastrointestinal diseases, including iron deficiency anemia, immune thrombocytopenia, and acute coronary syndromes6. H. pylori is a group I carcinogen that is responsible for more than 80% of gastric cancers and is the fourth leading cause of cancer-related deaths worldwide7,8. The prevalence of H. pylori infection is 40–75% in the global population; approximately 70% in developing countries and 25–50% in developed countries9. In 2017, H. pylori was included in the World Health Organization's "priority list of antibiotic-resistant bacteria" and was ranked top among the most common community-acquired infections10.
The efficacy of H. pylori eradication therapy is diminishing because of the rise of antimicrobial resistance. Clarithromycin (CLR), commonly used in triple therapy regimens, is an effective antibiotic for the treatment of H. pylori infection11; however, eradication failure has increased with the emergence of CLR-resistant strains. The first reported eradication rate for primary standard triple therapy in Japan was just over 85%. This then declined because of the spread of CLR-resistant H. pylori12. A recent meta-analysis of data from 54 countries showed a CLR-resistant strain prevalence of 27.5%. This resistance rate increased from 24.3% in 2010–2017 to 32.1% in 2018–202113. The differences in reported rates of CLR resistance result from differences in antibiotic use, patient compliance, access to healthcare, bacterial evolution and mutation, and other factors. Point mutations in 23S rRNA, specifically A2142G and A2143G in domain V, are associated with CLR resistance of H. pylori14,15,16. These mutations induce structural changes in the ribosomal binding site and reduce the affinity of CLR to 23S rRNA in the 50S rRNA subunit. Other rarely reported point mutations in 23S rRNA include; A2115G, T2117C, G2141A, T2182C, G2224A, C2245T, T2289C, C2611A, and T2717C17,18,19. Of these, A2115G, T2117C, G2141A, G2224A, C2245T, and T2289C mutations have not been confirmed. However, T2182C, C2611A, and T2717C are associated with low levels of CLR resistance20.
Amoxicillin (AMX), a β-lactam antibiotic, is widely used to treat H. pylori infection. Mutations in the genes encoding penicillin-binding proteins can reduce affinity for AMX, resulting in AMX resistance21,22, although the prevalence of AMX-resistant strains is comparatively low23. A meta-analysis demonstrated that the primary resistance rate of AMX was approximately 3% in the Asia–Pacific region24. However, the prevalence of AMX-resistant strains is gradually increasing because of mutations in PBP1A25,26,27. Primary AMX resistance rates have recently reached 13% in Japan28. The resistance rates vary by region, but there is a worrying global trend of increasing drug resistance23. A minimum inhibitory concentration (MIC) of > 0.125 μg/mL defines AMX resistance (European Committee on Antimicrobial Susceptibility Testing)29, and H. pylori with a MIC of ≥ 0.5 μg/mL is considered AMX-high resistance (AMXHR)30. Eradication failure rates were significantly higher in cases of AMXHR compared with cases with lower AMX resistance30. The use of MIC ≥ 0.5 μg/mL has been recommended as an index for judging therapeutic efficacy.
The treatment of H. pylori infection using CLR and AMX as primary eradication therapy has been widely employed. The success rate of primary eradication therapy that includes vonoprazan (a potassium-competitive acid blocker) instead of a proton pump inhibitor is currently acceptable, but the eradication failure rate is increasing because of the emergence of CLR-resistant and AMX-resistant H. pylori strains. In Japan, patients can receive insurance-covered primary eradication therapy without susceptibility testing. Susceptibility tests usually involve the laboratory examination of single isolate from patients.
H. pylori exhibits high genetic diversity, with DNA sequence polymorphism ranging from 2.7 to 8.0%31,32,33. Intraspecific genetic diversity produces diverse phylogenetic populations. This genetic diversity is generated through multiple mechanisms, including spontaneous point mutations, recombination with other bacteria, and intragenomic rearrangements involving mobile genetic elements or repetitive DNA sequences. These characteristics of H. pylori affect the heterogeneity of the community in the stomach. However, previous reports have commonly focused on findings derived from a single isolate obtained from a patient. The adaptability and flexibility of H. pylori allows the community to continuously adapt to changes in the gastric environment, leading to persistent infection and the emergence of considerable heterogeneity2. H. pylori diversity is an important phenomenon to consider when studying the pathogenesis of this bacterium and developing new treatments for H. pylori-associated diseases34.
The prevalence of heteroresistance to various antibiotics has been reviewed as impacting the reduced efficacy of eradication therapy with silent resistant isolates that were not discovered in clinical laboratory due to the low number of isolates tested35,36. A systematic review coupled with meta-analysis showed that the heteroresistance rate was 6.8% for CLR and 13.8% for metronidazole (MNZ)37. There is little literature on heteroresistance to AMX and sitafloxacin (STFX), and the rates of 3–19.6% for AMX and 0–18.2% for STFX are reported in unpublished data. However, these studies consider the heterogeneity of H. pylori in the stomach, usually being limited to the analysis of two or three isolates per patient, which is still controversial. The H. pylori community consisting of isolates with different properties adapts to the gastric environment as persistent infection. The heterogeneity of H. pylori in the stomach, particularly in primary eradication-failed patients, is uncertain. We therefore identified the heterogeneity of H. pylori in the stomach by susceptibility testing using multiple isolates from patients to characterize drug-resistant isolates in the H. pylori community. Furthermore, we identified mutations associated with CLR and AMX antibiotic resistance.
Materials and methods
Patients and samples
This study was approved by the Ethics Committee of Kochi University (approval number 27–35) and human samples were used in accordance with the guidelines of Kochi Medical University Hospital and the Declaration of Helsinki. Written informed consent was obtained from all enrolled patients. Multiple isolates were collected from 42 Japanese patients who underwent upper gastrointestinal endoscopy at Kochi Medical University Hospital. The 42 patients consisted of 17 males and 25 females aged 35–89 years (Table 1). Twenty-eight patients in whom primary eradication therapy with CLR and AMX failed were assigned to an eradication-failure group and 14 patients who had never received antibiotic eradication therapy were assigned to a treatment-naïve group. To assess the heterogeneity of H. pylori community within the stomach, 2–3 biopsy specimens were endoscopically collected from distinct regions (antrum, corpus, and the area close to a tumor region (tumor)). The specimens were subjected to histopathological analysis and to homogenization for bacterial culture. The homogenized biopsies were spread on selective plates for H. pylori (Vi HELICO AGAR, Eiken Chemical Co. Ltd, Japan) as primary selection step. We carefully obtained multiple colonies by detailed observations of individual properties such as growth speed, colony size and color, etc. in the primary selection step. The isolates were identified by gram-staining and PCR as previously described38 (Supplementary Table S1). At least five isolates were collected from each biopsy specimen of the stomach (antrum, corpus, and tumor). The multiple isolates used in this study per patient (Table 1). A total of 512 isolates (231, 241, and 40 isolates from antrum, corpus, and tumor, respectively) were isolated, consisting of 319 and 193 isolates from primary eradication-failed and treatment-naïve patients, respectively.
Bacterial strains and culture conditions
Helicobacter pylori
H. pylori strains, J99, 26695 and HPK5, clinical isolates and engineered recombinants, were used in this study (Table 2). H. pylori was grown on Brucella broth (Becton, Dickinson and Company, USA) agar plates (BB-plates) supplemented with 10% horse serum, vancomycin (10 μg/mL), and 1.4% agar (Wako Pure Chemical, Japan), at 37 °C under 10% CO2 (Sanyo CO2 incubator, Japan). Brucella broth supplemented with 10% horse serum (BB-liquid) was used when appropriate. Isolates were stored at − 80 °C until use.
Escherichia coli
E. coli DH5α (Nippon gene, Japan) was used to construct plasmids. Bacteria were cultured on Luria–Bertani broth (Nacalai Tesque, Japan) agar plates (LB-plates) supplemented with ampicillin (Amp) (50 μg/mL) and 1.4% agar at 37 °C under aerobic conditions.
Drug-susceptibility tests
The 512 isolates from the 42 patients were subjected to drug-susceptibility tests with four antibiotics, CLR, AMX, metronidazole (MNZ) and sitafloxacin (STFX), to obtain MICs. The E-test was used for CLR, AMX and MNZ, and the agar dilution method was used for STFX. Briefly, well-grown isolates were suspended in 1 mL of BB-liquid (to absorbance > 1.0 at OD600) and inoculated onto BB-plates with sterile cotton swabs. E-test strips (Biomerieux, Japan) were then placed on the plates according to manufacturer's instructions. These plates were incubated at 37 °C for 3–5 days. MIC values were determined, and antibiotic resistance was defined as; MIC ≥ 1 μg/mL for CLR, > 0.125 μg/mL for AMX, ≥ 16 μg/mL for MNZ and ≥ 0.5 μg/mL for STFX. Based on the drug-susceptibility tests, the 42 patients were classified into three categories: drug-heteroResistant/Sensitive (heteroR/S)-patients (in whom resistant and sensitive isolates coexist in the stomach), drug-homoresistant (homoR)-patients (in whom only resistant isolates are present in the stomach), and drug-homosensitive (homoS)-patients (in whom only sensitive isolates are present in the stomach).
PCR and cycle sequencing
Bacterial genomic DNA was extracted using a QIAprep Spin Miniprep Kit (QIAGEN, Germany) and used to PCR amplify 23S rRNA39 and pbp1A28. The PCR conditions and primers used are listed in Supplementary Table S1. The PCR amplicons were then purified using a Fast Gene Extraction Kit (Nippon Genetics Co. Ltd., Japan) and the expected product sizes of 1093 bp for 23S rRNA and 905 bp for pbp1A were confirmed. Amplicons were cycle sequenced using Big Dye Terminator (Applied Biosystems, USA) and a specific primer in 20 μL reaction mixtures. Ten μL of Xterminator solution and 45 μL of SAM solution (Applied Biosystems, USA) were added to the mixture, vigorously mixed (vortexed for 15 min), and then the mixture was applied to a DNA sequencer (3500xL Genetic Analyzer, 24chRUO, HITACHI, Japan) according to manufacturer's instructions.
Mutation analysis
We performed mutation analysis of 23S rRNA in 116 randomly selected isolates from 10 patients, including 65 isolates from all 5 CLR-heteroR/S-patients, 24 isolates from 2 CLR-homoR-patients, and 27 isolates from 3 CLR-homoS-patients. For amino acid analysis of PBP1A, 87 isolates were analyzed, including 48 isolates from all 4 AMX-heteroR/S-patients, 12 isolates from 1 AMX-homoR-patient and 27 isolates from 3 AMX-homoS-patients. The sequence of double strand was performed more than two times for each target region and the alignment was analyzed to confirm the homology by the Basic Local Alignment Search Tool (BLAST). BLASTn and BLASTp were used for nucleotide and amino acid sequences analyses based on Swiss-Prot database, respectively. Four (HPU27270.1, J99, 26695, and HPK5) and five (1061, 69A, J99, 26695, and HPK5) strains were used as reference strains for CLR and AMX, respectively.
Construction of plasmids
The bacterial strains and plasmids used are listed in Table 2. To identify mutations associated with AMX resistance, we constructed two plasmids (pE7/TOPO2.1 and pE50/TOPO2.1) for use in homologous recombination to generate six recombinants based on three H. pylori strains (J99, 26695 and HPK5). We used genomic DNA of two AMX-resistant isolates (E7AG3 and E50AG3) that possess G591K and A480V mutations in PBP1A, respectively, which are thought to be associated with AMX resistance. Briefly, PCR was performed using genomic DNA of the two isolates. Each amplicon was ligated into pCRTM2.1-TOPO® using a TOPO Cloning kit (Invitrogen, USA) to construct pE7/TOPO2.1 and pE50/TOPO2.1. Aliquots of the ligation mixture were used to transform E. coli DH5α, which were then spread on pre-warmed LB-plates containing 50 µg/mL Amp, X-Gal, and IPTG, and incubated overnight at 37 °C. We selected several white colonies and performed PCR to confirm the plasmids harboring the PCR fragments. Finally, PCR fragments amplified from pE7/TOPO2.1 and pE50/TOPO2.1 were confirmed by sequencing. We also constructed two plasmids to confirm involvement of the mutation associated with AMX resistance. We used genomic DNA of two further AMX-resistant isolates (E7BG5 and E50BG4), which possess the same amino acid sequences as E7AG3 and E50AG3 except they do not contain the G591K and A480V mutations. According to the method described above, two plasmids (pE7BG5/TOPO2.1 and pE50BG4/TOPO2.1) were constructed. These four plasmids were used for homologues recombination to generate 12 recombinants.
Homologous recombination
We performed homologous recombination to produce recombinants possessing the PBP1A G591K or A480V mutations using AMX-sensitive strains without such mutations (J99, 26695 and HPK5), as described previously40. Briefly, well-grown strains on BB-plates were inoculated into BB-liquid media to prepare high-concentration bacterial suspensions. An aliquot of plasmid DNA was added to 50 μL of bacterial suspension and dropped onto a BB-plate and incubated at 37 °C for 4–5 h. The drop was then spread using a sterile cotton swab and cultured for a few days. The bacteria that grew on the BB-plate were harvested, spread onto selective BB-plates supplemented with various concentrations of Amp, and cultured at 37 °C until colonies emerged. A few colonies for each of the 12 recombinants were subjected to the AMX-susceptibility test to determine MIC values. The mutations in PBP1A were confirmed by sequencing (Table 2).
Instead of plasmids, purified PCR products from E23AG1 and H11BG3 isolates were used in homologues recombination with CLR-sensitive strain J99 to determine whether the A1738G mutation found contributes to CLR resistance. However, we did not obtain recombinants as there was no growth on CLR plates. Therefore, using several colonies grown on BB-plates without CLR, we performed PCR-based single nucleotide polymorphism (SNP) designed to select the recombinants that exchanged for A1738G. Finally, recombinants with a sequencing-confirmed A1738G mutation were subjected to the CLR-susceptibility test.
Statistical analysis
Statistical analyses were conducted using SPSS statistical software package version 25.0 and p value ≤ 0.05 was considered statistically significance. The MIC values were validated by non-parametric Wilcoxon tests with medians and interquartile ranges.
Results
Drug-susceptibility testing of 512 isolates from 42 patients
Multiple isolates from each stomach of 42 patients were evaluated by drug-susceptibility tests with four antibiotics to demonstrate community heterogeneity. The drug-susceptibility test clarified the coexistence of drug-resistant and drug-sensitive isolates in a stomach. For CLR, AMX, MNZ and STFX, 12%, 10%, 33% and 31%, respectively, of all 42 patients were drug-heteroR/S-patients. Sixty-four percent of the 42 patients were drug-heteroR/S for at least one of the four drugs. Drug-homoR-patients and drug-homoS-patients were observed and are summarized in Fig. 1a and Table 1. Of the 28 patients in whom primary eradication with CLR and AMX failed, 11%, 14%, 18% and 25% were drug-heteroR/S for CLR, AMX, MNZ and STFX, respectively. Of the 14 treatment-naïve patients, 14%, 0%, 64% and 43% were drug-heteroR/S for CLR, AMX, MNZ and STFX, respectively. Fifty-four percent and 86% of patients in the eradication-failure group and the treatment-naïve group, respectively, were drug-heteroR/S for at least one of the four drugs.
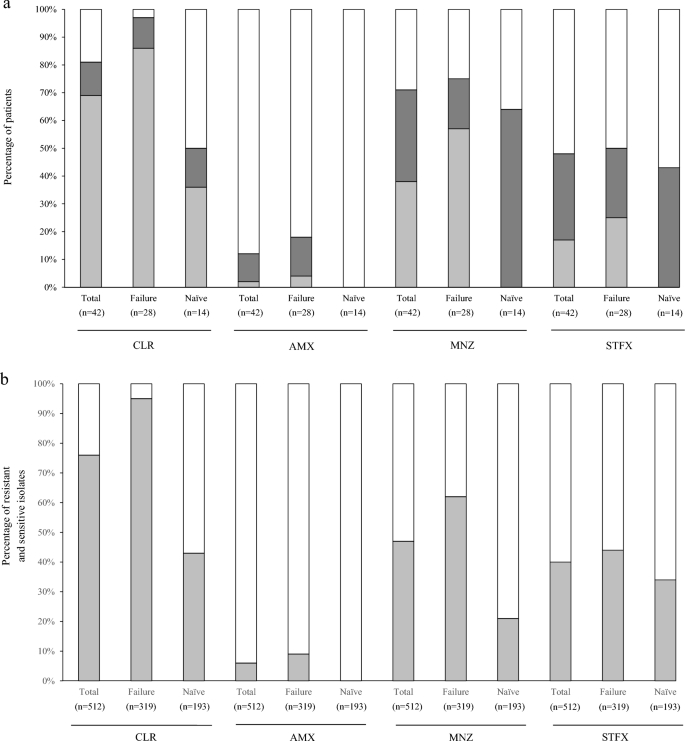
The proportions of patients and isolates based on drug-susceptibility test with four antibiotics. (a) The 42 patients are classified into three categories; drug-heteroR/S-patients, drug-homoR-patients and drug-homoS-patients. For CLR, the proportion of CLR-homoR-patients is considerable high in the primary eradication-failure group. CLR-homoS-patients dominate in the treatment-naïve group. CLR-heteroR/S-patients are slightly higher in treatment-naïve group than primary eradication-failure group. For AMX, AMX-homoR-patients and AMX-heteroR/S-patients are found in only primary eradication-failure group. For MNZ, MNZ-homoR-patients are found in only primary eradication-failure group and MNZ-heteroR/S-patients dominate in treatment-naïve group. For STFX, STFX-heteroR/S-patients are slightly higher in treatment-naïve group than primary eradication-failure group. STFX-homoR-patients are found in only primary eradication-failure group and STFX-homoS-patients dominate in two groups. light grey square, Drug-homoR-patients; grey square, Drug-heteroR/S-patients; white square, Drug-homoS-patients. (b) Total 512 isolates from 42 patients show the rates of resistant and sensitive isolates in 4 drugs. For CLR, the proportion of resistant isolates is high in primary eradication-failure group but nearly equal in treatment-naïve group. For AMX, resistant isolates are detected in only primary eradication-failure group, however, sensitive isolates dominate in the two groups. For MNZ, the proportion of sensitive isolates is small preponderance in primary eradication-failure group and sensitive isolates dominate in treatment-naïve group. For STFX, the proportion of sensitive isolates dominate in the two groups. light grey square, resistant isolates; white square, sensitive isolates. Failure primary eradication-failure, Naïve treatment-naïve, CLR clarithromycin, AMX amoxicillin, MNZ metronidazole, STFX sitafloxacin.
In the eradication-failure group 86% of patients were CLR-homoR. In the treatment-naïve group 36% were CLR-homoR-patients and 50% were CLR-homoS-patients. There was a similar prevalence of CLR-heteroR/S-patients in these two groups. AMX-homoR-patients and AMX-heteroR/S-patients were only observed in the eradication-failure group, indicating the consequence of primary eradication-failure. MNZ-homoR-patients were the most common patients in the eradication-failure group (at 57%) and MNZ-heteroR/S-patients were most common in the treatment-naïve group (at 64%). STFX-homoS-patients were the most common patients in the eradication-failure group (at 50%) and treatment-naïve group (at 57%). STFX-homoR-patients were only observed in the eradication-failure group. The susceptibility test using multiple isolates revealed that the infection rate of patients with drug-resistant isolates (drug-heteroR/S-patients and drug-homoR-patients) was higher than that for drug-homoR-patients, regardless of eradication treatment (Fig. 1a, Table 3). The evaluation of susceptibility test using a single isolate may not reflect the exact status of H. pylori infection in the human stomach. These findings emphasizes that susceptibility test using multiple isolates is importance and adequate method to evaluate the status of H. pylori infection in the stomach. Furthermore, 4, 9 and 19 patients were infected with isolates multi-resistant to 4, 3 and 2 drugs, respectively (Table 1). All four patients with isolates multi-resistant to all four drugs belonged to the eradication-failure group. The isolates multi-resistant to all four drugs coexisted with other isolates in the stomach.
CLR-resistant isolates accounted for 304 of the 319 isolates (95%) from eradication-failed patients and 83 of the 193 isolates (43%) from treatment-naïve patients (Fig. 1b). There were 29 (9%) AMX-resistant isolates in the eradication-failed patients and 0 (0%) in the treatment-naïve patients. For four drugs, AMX-sensitive isolates were most common among all 512 isolates, which is consistent with previous reports28. For MNZ, there were 198 (62%) and 41 (21%) resistant isolates in eradication-failed patients and treatment-naïve patients, respectively. Interestingly, MNZ-heteroR/S-patients were the most common patients (approximately 64%) among the treatment-naïve patients, but the prevalence of MNZ-resistant isolates was relatively low at 21%, indicating that MNZ-sensitive isolates dominated in MNZ-heteroR/S-patients. There were 139 (44%) and 66 (34%) STFX-resistant isolates in eradication-failed patients and treatment-naïve patients, respectively. STFX-sensitive isolates predominated in the two groups.
Drug-heteroR/S-patients
We performed detailed analysis on drug-heteroR/S-patients in which both drug-resistant and drug-sensitive isolates coexist in the stomach and these results were summarized (Fig. 2a, Table 4). A total of 65 isolates were isolated from all 5 CLR-heteroR/S-patients, consisting of 3 eradication-failed patients and 2 treatment-naïve patients. The detection rates of CLR-resistant isolates were higher than those of CLR-sensitive isolates in both two groups, regardless of eradication treatment. CLR-resistant isolates were dominant in the corpus in eradication-failure patients, while CLR-resistant isolates were evenly distributed among the different stomach regions in treatment-naïve patients (Fig. 2b).
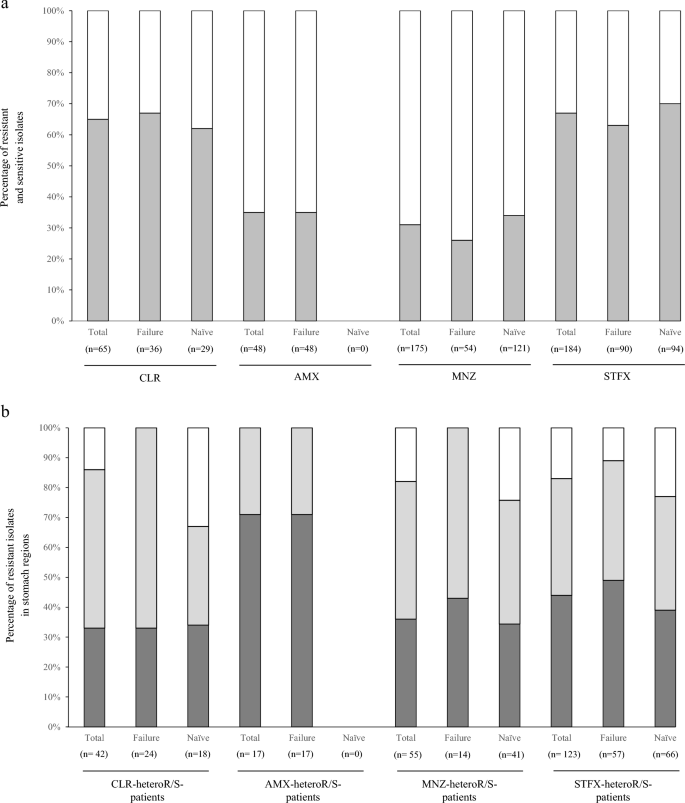
The characterization of isolates in the drug-heteroR/S-patients on four antibiotics. (a) The proportions of resistant and sensitive isolates in the drug-heteroR/S-patients. In CLR-heteroR/S-patients, resistant isolates dominate in the two groups. AMX-heteroR/S-patients are observed in only primary eradication-failure group, and resistant isolates are less than 40%. In MNZ-heteroR/S-patients, the proportions of sensitive isolates dominate in the two groups. Conversely, the proportions of resistant isolates dominate in the two groups of STFX-heteroR/S-patients. light grey square, resistant isolates; white square, sensitive isolates. (b) The distribution of resistant isolates in stomach regions of the drug-heteroR/S-patients. CLR-resistant isolates dominantly occupy in the corpus of the primary eradication-failed patients; however, the distribution is even across the stomach regions in treatment-naïve patients. Conversely, AMX-resistant isolates dominate in antrum of the primary eradication-failed patients. MNZ-resistant and STFX-resistant isolates are almost equivalent distribution across the different stomach regions, regardless of primary eradication treatment. grey square, antrum; light grey square, corpus; white square, tumor. Failure primary eradication-failure, Naïve treatment-naïve, CLR clarithromycin, AMX amoxicillin, MNZ metronidazole, STFX sitafloxacin.
The median MIC values of the 24 CLR-resistant isolates in eradication-failed patients were 2.5 and 48 μg/mL in the antrum and corpus, respectively. The MICs of 18 CLR-resistant isolates in treatment-naïve patients were 18, 24 and 24 μg/mL in the antrum, corpus, and tumor, respectively (Table 4). These findings indicated that CLR-resistant isolates with high MIC value emerged to become dominant in the corpus of eradication-failed patients.
We similarly analyzed 48 isolates, including 17 AMX-resistant and 31 AMX-sensitive isolates, from all 4 AMX-heteroR/S-patients that belonged to the eradication-failure group. AMX-resistant isolates dominated in the antrum. The median MIC values of these 17 AMX-resistant isolates were 0.25 and 0.25 μg/mL in the antrum and corpus, respectively. Interestingly, this finding contrasted with CLR resistance in primary eradication-failure patients.
We analyzed 175 isolates, including 55 MNZ-resistant and 120 MNZ-sensitive isolates, from all 14 MNZ-heteroR/S-patients, including 5 eradication-failed patients and 9 treatment-naïve patients. MNZ-resistant isolates were 26% and 34% of all isolates in eradication-failed patients and treatment-naïve patients, respectively. The MNZ-resistant isolates were evenly distributed across all stomach regions in the two groups. The median MICs of MNZ-resistant isolates were high for those from the corpus and antrum of eradication-failed patients and treatment-naïve patients, respectively.
We analyzed 184 isolates, including 123 STFX-resistant and 61 STFX-sensitive isolates, from all 13 STFX-heteroR/S-patients consisting of 7 eradication-failed patients and 6 treatment-naïve patients. STFX-resistant isolates were 63% and 70% of all isolates in eradication-failed patients and treatment-naïve patients, respectively. The STFX-resistant isolates were evenly distributed among the different stomach regions with similar median MIC values in the two groups.
Next, we compared the MIC values of drug-resistant isolates between drug-heteroR/S-patients and drug-homoR-patients (Table 4). CLR MIC values were highest in the isolates from the corpus in CLR-heteroR/S-patients of the eradication-failure group. AMX MIC values were highest in the isolates from the antrum of AMX-homoR-patients of the eradication-failure group. MNZ MIC values were higher in MNZ-homoR-patients than in MNZ-heteroR/S-patients for the two groups. STFX MIC values did not differ between STFX-heteroR/S-patients and STFX-homoR-patients of the two groups.
Mutations in genes involved in CLR and AMX resistance
Nucleic acid analysis of 23S rRNA with respect to CLR resistance
We randomly selected isolates to investigate mutations in 23S rRNA related to CLR resistance. A total 116 isolates from 10 patients consisting of all 5 CLR-heteroR/S-patients, 2 CLR-homoR-patients and 3 CLR-homoS-patients were analyzed. Sixty-six CLR-resistant and 50 CLR-sensitive isolates were subjected to sequencing. CLR resistance-related point mutations in 23S rRNA, A2142G or A2143G, were detected in 65 of 66 CLR-resistant isolates (98%). A2143G was found in 2 of 50 CLR-sensitive isolates. A2142G and A2143G were detected in 5% (3/66) and 94% (62/66) of CLR-resistant isolates, respectively. Next, A1738G mutation was found in 44% (29/66) of CLR-resistant isolates, but only one of 50 CLR-sensitive isolates. Furthermore, A1738G was found in 47% of 62 CLR-resistant isolates harboring A2143G. There was no CLR-resistant isolate harboring single A1738G mutation, leading to the relationship between A1738G and A2143G. We therefore compared the MIC values between 33 isolates with A2143G and 29 isolates with A1738G and A2143G. The median MICs were 32 μg/mL for A2143G and 16 μg/mL for A1738G and A2143G, indicating reduced MICs for isolates with the two mutations.
Amino acid analysis of PBP1A with respect to AMX resistance
A total of 87 isolates from 8 patients consisting of all 4 AMX-heteroR/S-patients, 1 AMX-homoR-patient, and 3 AMX-homoS-patients were subjected to sequencing. Among the 87 isolates, 29 were AMX-resistant and 58 were AMX-sensitive. Seventeen mutations resulting in amino acid changes were detected in the 87 isolates and were categorized into three MIC levels: AMX-sensitive (≤ 0.125), AMX-low resistance (AMXLR) (0.125–0.5) and AMX-high resistance (AMXHR) (≥ 0.5) (Table 5). Among the 17 mutations, two, A480V and G591K, were observed in AMXHR only. We therefore evaluated MIC values in recombinant strains that possess these two mutations. In addition, the number of mutations detected was evaluated for association with the three levels of AMX resistance. A statistically significant difference (p < 0.01) was observed between AMX-sensitive and AMXHR isolates (Fig. 3), indicating that the increased number of mutations contributes to the increased MIC value of AMX.
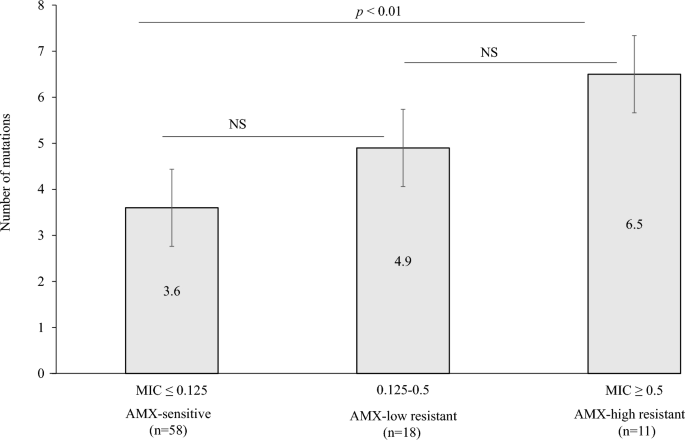
Correlation between the MIC of AMX (μg/mL) and the number of mutations in penicillin-binding protein 1A. NS not significant, AMX amoxicillin.
Evaluation of AMX and CLR MIC values using H. pylori recombinant strains
Six recombinants based on three AMX-sensitive H. pylori strains were produced by homologous recombination using two plasmids harboring either PBP1A G591K or A480V mutations. E7.J99 and E50.J99 recombinants strains were produced from strain J99 and possessed either G591K or A480V mutations, respectively. We performed drug-susceptibility tests with clones from all six recombinants and showed that the MIC values of all six recombinants were obviously increased (Table 2). We also showed that the MIC value of recombinants with G591K was higher than that for recombinants with A480V. The G591K mutation more effectively converted the AMX-sensitive strain to the AMX-resistant strain compared with the A480V mutation. We similarly prepared six more recombinants possessing the same amino acid sequences but without G591K and A480V. Drug-susceptibility tests demonstrated the MIC values of all six recombinants to be slightly increased but not up to the level of AMX-resistance. Taken together, these findings confirmed the G591K and A480V mutations to be associated with AMX resistance. We also assessed whether the A1738G mutation contributes to CLR resistance using recombinant strains derived from CLR-sensitive strain J99 lacking the A1738G mutation. The recombinants in which A1738G was replaced were confirmed by PCR and sequencing. Drug-susceptibility tests with these recombinants revealed their MICs to be unchanged compared with the strain J99 lacking the A1738G mutation.
Discussion
The failure to eradicate H. pylori infection with common antibiotics is increasing because of drug-resistant isolates. H. pylori has high genetic diversity and produces a community containing many mutants, which adapt to the microenvironments of an individual's stomach to achieve persistent infection. However, single isolate is usually used in studies concerned with H. pylori infection and for clinical assessment. Prevalence of heteroresistance (drug-heteroR/S) to antibiotics is a multifaced issue for clinical practices and research. In a limited number of literatures, the heteroresistance rates between studies are difference and still controversial. The different rates across diverse populations and geographical regions emphasize the need of methodology to understand the exact situation of H. pylori in the stomach37,41. Furthermore, patients' information such as eradication therapy and manifestation is unclear in previous reports. In this study, we used multiple isolates from individual patients to determine the community heterogeneity in individual stomachs. We showed that the rate of heteroresistance was 12%, 10%, 33% and 31% for CLR, AMX, MNZ, and STFX, respectively. The heteroresistance rate is higher than those of previous reports. In addition, the rates differed between primary eradication-failure and treatment-naïve, and within gastric regions of the stomach. These provided important insight into the characteristics of H. pylori infection for clinical practices.
Among the 42 patients examined, 64% were drug-heteroR/S for at least one of the four drugs tested, and these rates were 54% and 86% in the 28 primary eradication-failed patients and 14 treatment-naïve patients, respectively. This indicates that H. pylori community heterogeneity is more common than expected. Among the 28 patients in whom primary eradication-failed using CLR and AMX, there were 11%, 14%, 18% and 25% drug-heteroR/S-patients for CLR, AMX, MNZ and STFX, respectively. The prevalence of drug-heteroR/S-patients was lower for CLR and AMX compared with MNZ and STFX. Among the 14 treatment-naïve patients, there were 14%, 0%, 64% and 43% drug-heteroR/S-patients for CLR, AMX, MNZ and STFX, respectively. The prevalence of drug-heteroR/S-patients was higher among treatment-naïve patients for CLR, whereas for AMX, it was higher among primary eradication-failed patients. However, interestingly, CLR-homoR-patients and AMX-homoS-patients predominated among the primary eradication-failed patients. In contrast, MNZ-heteroR/S-patients were the most common among the treatment-naïve patients. In this study, most treatment-naïve patients had malignancies, such as gastric cancer and mucosa-associated lymphoid tissue lymphoma, indicating an effect of the cancerous stomach microenvironment on MNZ resistance. The rdxA and frxA genes are ...
Comments
Post a Comment