Lyophilized cell-free supernatants of Limosilactobacillus fermentum T0701 exhibited antibacterial activity against ... - Nature.com
Abstract
Helicobacter pylori is a prominent gastrointestinal pathogen associated with various gastrointestinal illnesses. It presents substantial health risks due to its antibiotic resistance. Therefore, it is crucial to identify alternative treatments for H. pylori infections. Limosilactobacillus spp exhibit probiotic properties with beneficial effects in humans; however, the mechanisms by which it counteracts H. pylori infection are unknown. This study aimed to evaluate the potential of Limosilactobacillus fermentum T0701 lyophilized cell-free supernatants (LCFS) against H. pylori. The LCFS has varying antimicrobial activities, with inhibition zones of up to 10.67 mm. The minimum inhibitory concentration and minimum bacterial concentration of LCFS are 6.25–25.00 mg/mL and 6.25 mg/mL to > 50.00 mg/mL, respectively, indicating its capability to inhibit H. pylori. There is morphological damage observed in H. pylori treated with LCFS. Additionally, H. pylori adhesion to AGS cells (human gastric adenocarcinoma epithelial cells) reduces by 74.23%, highlighting the LCFS role in preventing bacterial colonization. Moreover, LCFS exhibits no cytotoxicity or morphological changes in AGS cells, and with no detected virulence or antimicrobial resistance genes, further supporting its safety profile. L. fermentum T0701 LCFS shows promise as a safe and effective non-toxic agent against H. pylori, with the potential to prevent gastric colonization.
Similar content being viewed by others

A Gram-negative-selective antibiotic that spares the gut microbiome
Reinforcement of the intestinal mucosal barrier via mucus-penetrating PEGylated bacteria
Pseudomonas aeruginosa breaches respiratory epithelia through goblet cell invasion in a microtissue model
Introduction
Helicobacter pylori is a gram-negative, microaerophilic, motile, spiral-shaped bacterium that predominantly colonizes the human gastric mucosa and plays a pivotal role in a wide spectrum of gastrointestinal pathologies, including asymptomatic to chronic gastritis, peptic ulcers, duodenal ulcers, mucosa-assisted lymphoid tissue lymphoma, and gastric cancer1,2. H. pylori infection is prevalent globally, affecting almost 50% of the world's population, with transmission often associated with the fecal–oral pathways2. From 2015 to 2022, the global prevalence of H pylori infection was 43.7% in adults and 34.4% in children and adolescents3. Moreover, in 1994, H. pylori was categorized as a Group 1 carcinogen by the World Health Organization International Agency for Research on Cancer4.
The pathogenesis of H. pylori involves a sophisticated mechanism that allows it to survive and thrive in the highly acidic environment of the stomach, such as flagella, urease, and toxins. Additionally, two major virulent factors of H. pylori have been reported to induce pathogenesis: cytotoxin-associated gene A (CagA), which is delivered to host cells via the type IV secretion system, and vacuolating cytotoxin A (VacA). These virulence factors disrupt cell signaling and induce inflammation, leading to tissue damage and ulcer formation. Chronic inflammation triggered by persistent infection can contribute to the development of atrophic gastritis and increase the risk of gastric cancer1,2,5. Currently, the main strategy for eradicating H. pylori involves a combination of oral antibiotics, proton pump inhibitors (PPIs), and bismuth agents. However, this treatment is not always effective; in 10–20% of cases, H. pylori is not eradicated after treatment, leading to recurrent H. pylori infection. Moreover, the antibiotic resistance rate of H. pylori has increased and the adverse effects of eradication therapy can be severe. Therefore, there is an urgent need to develop novel, inexpensive, alternative, and implementable strategies to combat H. pylori infection, such as new anti-H. pylori drugs.
Probiotics are live microorganisms with low or no pathogenicity, which confer health benefits to the host when administered in adequate amounts6. Currently, the clinical benefits of probiotics are widely accepted, and their therapeutic applications include disorders, such as diarrhea, antibiotic-associated diarrhea, functional digestive involvement, inflammatory bowel disease, cardiovascular diseases, allergic reactions, and cancer. Probiotic activity has been associated with Bifidobacteria, Saccharomyces boulardii and lactic acid bacteria, such as Limosilactobacillus (previous name: Lactobacillus) spp., Lactococcus spp., Carnobacterium spp., Enterococcus spp., Streptococcus spp., Pediococcus spp., and Propionibacterium spp. Probiotics can produce secondary metabolites, such as short-chain fatty acids, exopolysaccharides, organic acids, hydrogen peroxide (H2O2), and bacteriocins, which is a key mechanism through which these beneficial bacteria exert their health effects, including enhancing gut health, supporting the immune system. Moreover, probiotics can inhibit various pathogens, such as H. pylori7, Clostridioides difficile8, Vibrio parahaemolyticus9, Escherichia coli O157: H710, and Staphylococcus aureus11.
We previously isolated Limosilactobacillus fermentum strain T0701 from fermented palm sap in the Songkhla Province of Southern Thailand. L. fermentum T0701 showed high inhibitory activity against the growth of Acinetobacter baumannii, E. coli, Listeria monocytogenes, Salmonella typhi, S. enteritidis, S. flexneri, S. aureus, and E. faecalis, and met the criteria for a potential probiotic12,13. However, information on its antibacterial activity against H. pylori is lacking. Therefore, the present study aimed to evaluate the potential of lyophilized cell-free supernatants (LCFS) of L. fermentum T0701 against H. pylori.
Results
Determination of minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) of LCFS
L. fermentum T0701 LCFS showed varied antimicrobial activities against H. pylori ATCC43504 and five clinical H. pylori isolates (Table 1). The inhibition zones were 0.00 ± 0.00 and 10.67 ± 0.58 mm. The MIC and MBC of L. fermentum T0701 LCFS against H. pylori were 6.25–25.00 mg/mL and 6.25 mg/mL to > 50.00 mg/mL, respectively. This suggests that L. fermentum T0701 LCFS protects against H. pylori.
Scanning electron microscopy (SEM)
The effect of the LCFS on the morphology of H. pylori was observed using SEM. The result showed that the normal bacterial cells (regular shapes and homogenous cell surface) appeared in the control groups of the H. pylori (Fig. 1A) and clinical H. pylori isolates (Fig. 1C). Notably, both H. pylori ATCC43504 and clinical H. pylori isolates treated with 1 × MIC of L. fermentum T0701 LCFS showed damaged cells compared with that in the control (Fig. 1B,D). The bacterial cells exhibited a rough, pitted, and irregular appearance along with a modified cell structure. Additionally, certain cells appeared damaged and displayed missing or ruptured membranes. Our findings suggest that the LCFS of L. fermentum T0701 interferes with the disruption of H. pylori, leading to the release of intracellular contents.
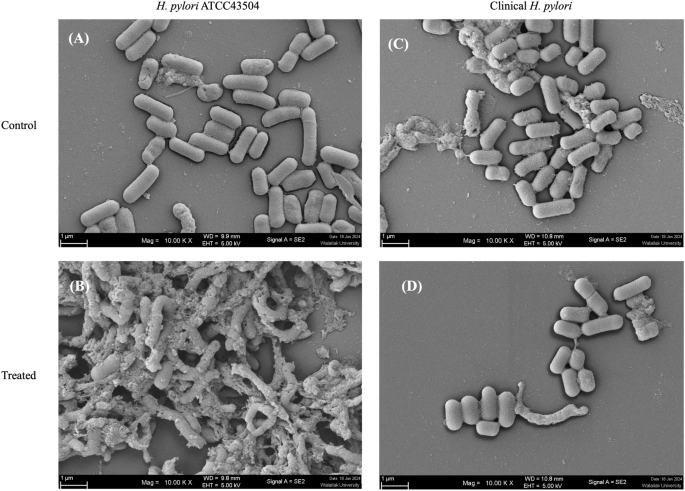
Morphology of H. pylori ATCC43504 (A,B) and clinical H. pylori (C,D) after treatment with LCFS of L. fermentum T0701 observed by SEM. The bacterial cells were treated with the LCFS at MIC. MRS was used as a negative control. Magnifications were revealed 10,000×. LCFS lyophilized cell-free supernatant, MIC minimum inhibitory concentration, MRS de Man, Rogosa and Sharpe.
Cell viability assay
The results of the in vitro toxicity evaluation of AGS cells using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay are shown in Supplementary Table 1. The results indicated that 0.625–10 mg/mL of L. fermentum T0701 LCFS exhibited nontoxic effects against AGS cells. The cell viability was 91.52 ± 1.10% to 118.58 ± 12.10%. Thus, the LCFS was considered safe.
Anti-adhesion of H. pylori to AGS cells by LCFS
We investigated whether L. fermentum T0701 LCFS could inhibit the adhesion of H. pylori to AGS cells. The results showed that the L. fermentum T0701 LCFS significantly reduced H. pylori adhesion to AGS cells by 74.23 ± 0.60% when compared with that with H. pylori infection alone (Supplementary Fig. 1). Moreover, de Man, Rogosa and Sharpe (MRS) medium did not inhibit H. pylori adhesion. These results suggest that L. fermentum T0701 LCFS adheres H. pylori to AGS cells.
Effect of co-culture of LCFS and H. pylori on AGS cells
To compare the effect of L. fermentum T0701 LCFS on H. pylori infection in AGS cells, AGS cells were co-infected with H. pylori and LCFS for 24 h. After infection, AGS cells were examined under inverted and holotomographic microscopes. The results showed that the morphology of AGS cells was relatively normal in L. fermentum T0701 LCFS-treated cells (Fig. 2C) compared with that of the negative control (Fig. 2A). AGS cells were round and became spherical when treated with H. pylori ATCC43504 (Fig. 2B). Notably, AGS cells treated with a co-culture of LCFS and H. pylori remained stable and cell rounding decreased (Fig. 2D) when compared with AGS cells treated with H. pylori. The morphology of AGS cells was observed under a holotomographic microscope. We found that the treatment of AGS cells with H. pylori led to changes in the morphology of the cells when compared with normal cells. The cells formed debris, which resulted in cell death. AGS cells co-cultured with LCFS and H. pylori were slightly damaged. However, it was more normal than that in AGS cells treated with H. pylori. Furthermore, F-actin detection was detected using immunofluorescence. The results showed that AGS cells in the control group exhibited a typical F-actin cytoskeleton and an imbibed nucleus similar to those of normal cells (Fig. 3A). AGS cells treated with H. pylori showed a loss of F-actin cytoskeleton-mediated interconnections between cells and condensed nuclei, indicating the initial stage of apoptosis. The cells became rounded and tight junctions were disrupted (Fig. 3B). The images of AGS cells with L. fermentum T0701 LCFS were similar to those of the control group, and F-actin showed a normal morphology (Fig. 3C). Moreover, The AGS cells treated with co-culture of LCFS and H. pylori were less damaged like normal morphology when compared with AGS cells that were treated with H. pylori (Fig. 3D). Overall, these results suggested that L. fermentum T0701 LCFS reduced the effects of H. pylori by inhibition.
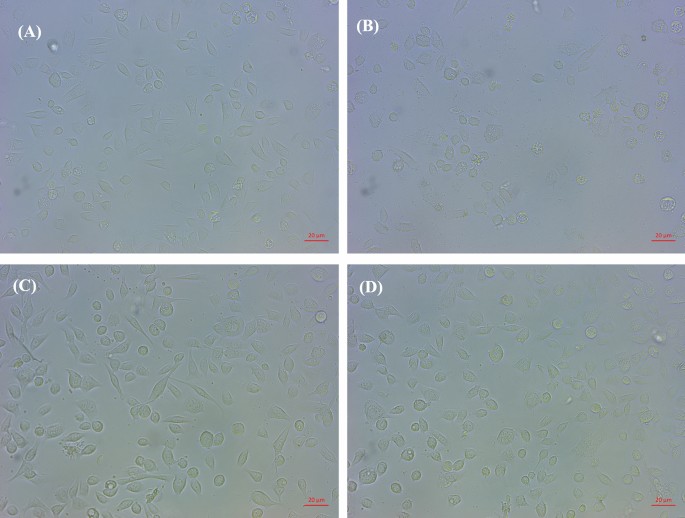
Comparison of cytotoxic effects of individual H. pylori ATCC43504, LCFS of L. fermentum T0701, and co-culture of the LCFS and H. pylori on AGS cells. (A) AGS cells, (B) AGS cells treated with H. pylori ATCC43504, (C) AGS cells treated with LCFS of L. fermentum T0701, (D) AGS cells treated with co-culture of LCFS and H. pylori. LCFS lyophilized cell-free supernatant.
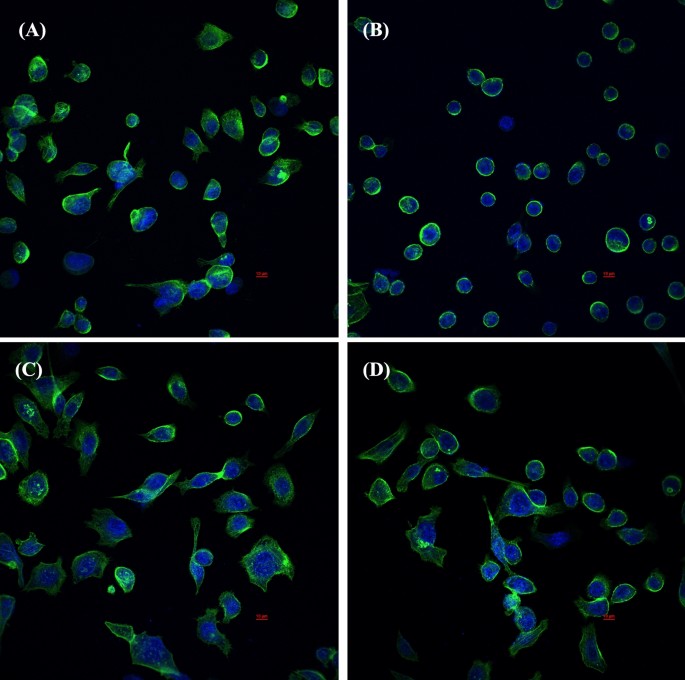
Immunofluorescence images obtained by confocal scanning laser microscopy of AGS cells after 24 h with individual H. pylori ATCC43504, LCFS of L. fermentum T0701, and co-culture of the LCFS and H. pylori on AGS cells. (A) AGS cells, (B) AGS cells treated with H. pylori ATCC43504, (C) AGS cells treated with LCFS of L. fermentum T0701, (D) AGS cells treated with co-culture of LCFS and H. pylori. DAPI-stained nucleus (blue, excited at 405 nm). F-actin stained with Phalloidin-Alexa-Fluor-488 probe (green, excited at 490 nm). LCFS lyophilized cell-free supernatant.
In silico safety evaluation and bacteriocin identification
In our analysis, we utilized the ABRICATE tool, a widely used bioinformatics software, to detect virulence genes by searching the VFDB (Virulence Factor Database). We applied stringent criteria with a Minimum DNA %identity of 80 and Minimum DNA %coverage of 80 during the examination. Despite these parameters, we did not identify any virulence genes in the analyzed samples. Furthermore, analysis of various biological features of this strain revealed predominant functions related to carbohydrates, amino acids, nucleotides, cofactors, and stress responses. Although these genes are commonly acknowledged as virulence factors in pathogens that support their survival in the host environment under physiological stress and facilitate adaptation to diverse conditions, the lack of such genes in T0701 addresses safety concerns. The absence of genes associated with virulence factors in strain T0701 suggested no harm to humans, confirming the lack of virulence genes (Table 2 and Fig. 4).
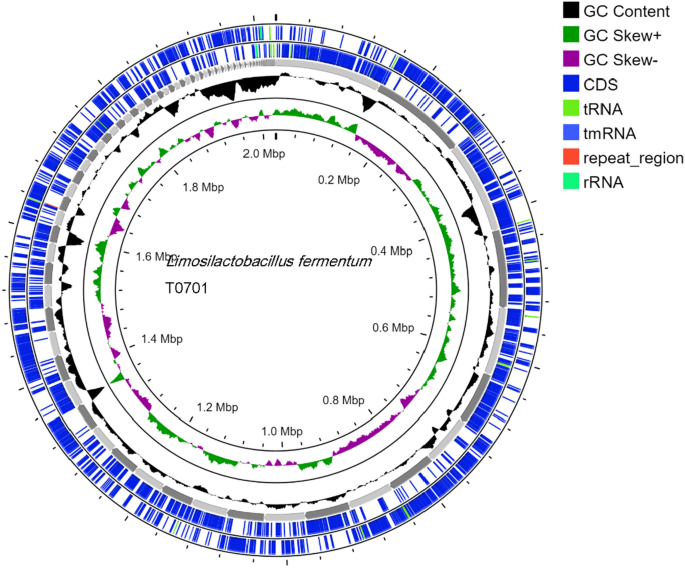
Circular genome map of Limosilactobacillus fermentum T0701.
No antimicrobial resistance genes were identified in the ResFinder database, which aligned with previous safety assurances. Antibiotic susceptibility testing confirmed T0701 susceptibility to a range of antibiotics, including chloramphenicol, kanamycin, and penicillin. Additionally, examination of the T0701 strain using the MGEFinder database revealed four insertion sequences without encoded genes, indicating the potential absence of transmission capability (Table 2 and Fig. 4). L. fermentum T0701 contains one intact and one incomplete phage. It contained two prophage regions (Supplementary Table 2).
We manually performed a BLASTP search against the BAGEL4 database to identify bacteriocins within the genome. Our investigation showed that the three proteins encoded by the T0701 strain displayed similarity to bacteriocin-like proteins, with a coverage of 100% and similarity of over 79%, as outlined in Supplementary Table 3. Their potential as targeted antimicrobial agents, particularly in inhibiting the H. pylori pathogen.
Discussion
H. pylori is the most common chronic bacterial infection worldwide and the greatest risk factor for gastric cancer. It remains the world's leading cause of cancer-related deaths. Moreover, it can cause chronic gastritis, peptic ulcers, and mucosa-associated lymphoid tissue lymphomas. In recent years, the use of PPIs in combination with antibiotics has significantly improved H. pylori treatment. However, there are substantial challenges in managing H. pylori infections owing to the increasing incidence of antibiotic resistance and the emergence of drug-resistant strains14. Probiotics and postbiotics are being explored as alternative therapeutic options for the treatment and prevention of H. pylori infections. Therefore, this study focused on evaluating the antibacterial potential of the LCFS of L. fermentum T0701 against H. pylori.
Probiotics are dietary supplements that consist of live, nonpathogenic microorganisms when administered in adequate quantities and are known to enhance the health status of the host6. Moreover, a postbiotics are defined as, "any factor resulting from the metabolic activity of a probiotic or any released molecule capable of conferring beneficial effects to the host in a direct or indirect way," with various therapeutic applications, especially antimicrobial effects, by using different mechanisms15. In our previous report12, the LCFS of L. fermentum T0701 isolated from fermented palm sap exhibited antimicrobial, antibiofilm, biofilm eradication, antioxidant, and anti-inflammatory activities by reducing nitric oxide production. In the present study, The L. fermentum T0701 LCFS strongly inhibited H. pylori, as revealed by agar well assays, followed by MIC and MBC assays. Various probiotics exert inhibitory effects on H. pylori through distinct mechanisms16, including L. johnsonii No. 108817, L. pentosus SLC13, and L. gasseri BCRC14619T18. Other reports have shown that L. rhamnosus GMNL-74, L. L. acidophilus and GMNL-185 can inhibit H. pylori infection and exhibit anti-inflammatory activities19. L. fermentum strains are known to have beneficial effects on human health20. They synthesize a range of secondary metabolites with antimicrobial properties, including bacteriocins, organic acids, ethyl alcohol, short-chain fatty acids, and hydrogen peroxide21. These peptides, which are generated by ribosomes, can disrupt the cell membrane or cause breakdown of the cell wall. However, the specific mechanism of action of some of these peptides remains unclear20. The L. fermentum T0701 LCFS destroyed the cell wall of H. pylori as observed using SEM.
In this study, we found that L. fermentum T0701 carries the lantibiotic immunity ABC transporter MutE/EpiE family permease subunit, lantibiotic protection ABC transporter ATP-binding protein, and the nisin biosynthesis regulatory protein NisR. The lantibiotic immunity ABC transporter MutE/EpiE family permease subunit is not directly involved in antimicrobial activity, but plays a crucial role in providing immunity to the host organism against the lantibiotic it produces. l-Antibiotics are a class of peptide antibiotics produced by certain bacteria that are highly effective against a broad range of bacterial pathogens. These antibiotics function by binding to the cell walls of target bacteria, disrupting cell wall synthesis or forming pores in the bacterial membrane, leading to cell death22,23. The lantibiotic protection ABC transporter ATP-binding protein is a key component of the self-protection mechanism of lantibiotic-producing bacteria, enabling them to safely produce lantibiotics that inhibit pathogens without the protein itself directly engaging in pathogen inhibition24. Although NisR itself does not inhibit pathogens, its regulation of nisin production is critical for the antimicrobial defense strategy of L. lactis. By controlling nisin production, NisR indirectly contributes to the inhibition of pathogenic bacteria in the environment where L. lactis resides. Once produced and secreted, nisin can bind to the cell wall precursors of susceptible bacteria, form pores in their membranes, and initiate cell death, thereby inhibiting the growth of pathogens25.
Furthermore, L. fermentum is acknowledged as be safe and listed in the official registers of European, American, and Chinese food safety authorities26,27,28. Prophages are commonly found in probiotic strains utilized in dairy fermentation, including Lactococcus, Bifidobacterium, and Limosilactobacillus29. Prophages are frequently detected in Limosilactobacillus strains, with a typical range of one to five prophages per genome30,31. This suggests that prophages are prevalent in the genomes of probiotic bacteria and emphasizes their significance in the context of dairy fermentation and related applications. In addition, L. fermentum T0701 did not carry antimicrobial resistance or virulence genes, suggesting that it is safe.
The adhesion of H. pylori to epithelial cells is a critical step in its colonization and infection of the human stomach. Most of these connections are facilitated by outer membrane proteins (OMPs) that function as adhesins. The genome of H. pylori has approximately 30 genes encoding OMPs2,16. The present study showed that L. fermentum T0701 LCFS reduced the adhesion of H. pylori to AGS cells. Similarly, Limosilactobacillus and S. boulardii, can prevent H. pylori from adhering to gastric epithelial cells by competing for binding sites, such as asialo-GM1 and sulfatide receptors19. Other probiotics such as L. acidophilus, L. johnsonii, and L. salivarius also exhibit similar properties, preventing H. pylori colonization through specific adhesion molecules19. Other studies have shown that cell-free supernatants of L. pentosus SLC13, L. rhamnosus LGG, and L. gasseri BCRC 14619 T can reduce H. pylori adhesion to GES-1 cells by 54.2%, 52.4%, and 38.0%, respectively18. Thus, the anti-adhesion effect is crucial for inhibiting H. pylori colonization and stomach infections, providing a potential complementary approach to traditional H. pylori eradication therapies.
Upon entry into cells, CagA can alter cell signaling pathways, promote inflammation, and potentially contribute to carcinogenesis. VacA causes cellular damage by forming pores in the cell membrane, leading to cell death and contributing to the pathology of H. pylori-associated diseases2,5,32. In the previous study, L. rhamnosus JB3 had multiple mechanisms to disrupt H. pylori pathogenesis and the cellular responses of AGS cells caused by infection, to combat the infection33. L. reuteri 2892 exhibited protective properties against H. pylori-induced gastritis in animal models by effectively suppressing the expression of virulence factors in the gastric mucosa34. Another study demonstrated that L. fermentum inhibits H. pylori in AGS cells through several key actions; L. fermentum UCO-979C has been shown to beneficially modulates the innate immune response triggered by H. pylori infection and reduce the adhesion of H. pylori to gastric epithelial cells. It achieves this by substantially reducing the production of inflammatory cytokines and chemokines while increasing the levels of immunoregulatory cytokines and reducing cell damage35. Moreover, L. fermentum MN-LF23 reduces H. pylori infection in infected mice and ameliorates H. pylori-induced gastric mucosal damage and lymphocyte infiltration36. Similarly, the results of our study showed that L. fermentum T0701 LCFS may secrete antibacterial agents that reduce the cytotoxic effects of H. pylori protection of AGS cells.
To the best of our knowledge, L. fermentum T0701 LCFS exhibited significant anti-H. pylori activity, inhibition of bacterial adhesion to AGS cells, and bactericidal properties. Moreover, it is safe and non-toxic to AGS cells, indicating its potential as a therapeutic agent against H. pylori infection. These findings suggest that LCFS could be an effective non-antibiotic strategy for managing H. pylori infections, potentially reducing the burden of antibiotic resistance and offering a complementary approach to current treatment regimens. Further research is warranted to identify the specific molecules responsible for anti-H. pylori activity and to explore the clinical potential and mechanisms underlying L. fermentum T0701 LCFS effects against H. pylori.
Methods
Bacterial strains and culture conditions
L. fermentum T0701 was isolated and characterized as a potential probiotic in a previous study13. Briefly, the isolate was grown in MRS broth (HiMedia, Mumbai, India) at 37 °C for 24 h. After that, all isolates were stored at − 80 °C in 30% (v/v) glycerol (Sigma, Steinheim, Germany) until further use.
H. pylori ATCC43504 strain was purchased from the American Type Culture Collection and 10 clinical H. pylori isolates were collected from Songklanagarind hospital. These strains were cultured on blood agar (HiMedia) containing 7% horse blood, and the agar plates were incubated at 37 °C for 48 h under microaerophilic conditions. The colonies were transferred to brain heart infusion broth (HiMedia) and incubated at 37 °C for 18 h. Each strain was stored at − 80 °C in brain heart infusion broth with 30% glycerol until further use.
Preparation of cell-free supernatants (CFS) and lyophilization
CFSs were prepared according to the method described by Sornsenee et al.12. L. fermentum T0701 was cultured in 250 mL of MRS broth and incubated at 37 °C for 24 h under anaerobic conditions. The bacterial cultures were then centrifuged at 10,000×g. The supernatant was sterile filtered using a 0.2 µm filter (Sigma, Steinheim, Germany). CFS of L. fermentum T0701 and only MRS medium as MRS control were frozen at − 80 °C for 24 h. The samples were lyophilized (Lyophilization Systems, Inc, USA) from − 40 to − 30 °C, 0.2 mbar. The entire freeze-drying process was performed in 24 h, and the freeze-dried powders were stored at − 20 °C. They were then rehydrated with sterile deionized water prior to use.
Agar well-diffusion assay
This assay was performed in accordance with Romyasamit, et al.8, with minor modifications. Briefly, LCFS of L. fermentum T0701 were resuspended in sterile deionized water (100 mg/mL). Each H. pylori ATCC43504 and 5 clinical H. pylori isolates (1.5 × 108 CFU/mL) were spread on blood agar containing 7% horse blood and were cut out of the agar using sterile Pasteur pipettes. Then 50 μL of LCFS were added into the agar wells. The plates were incubated at 37 °C for 72 h under microaerophilic conditions and were inspected for the presence of inhibition zones. The antibacterial activity was expressed as the mean of inhibition diameters (mm) produced by LCFS. The tests were performed in triplicate.
Determination of MIC and MBC
The antimicrobial efficacy of LCFS against H. pylori ATCC43504 was evaluated employing the microdilution method in 96-well plates, following the Clinical and Laboratory Standards Institute 2022 guidelines37. 100 µL of serial dilution of LCFS (100–3.125 mg/mL) were prepared in Mueller Hinton broth (MHB) (HiMedia). The H. pylori ATCC43504 suspensions were adjusted to McFarland turbidity standard 2 and then 100 µL was inoculated into each well. The plates were incubated at 37 °C for 72 h under microaerophilic conditions. To determine the MIC, the colorimetric dye resazurin was used, which serves as an indicator of cell viability. After the incubation period, 20 µL of resazurin solution (0.01% w/v in sterile PBS was added to each well). The plates were then incubated for 4 h. The MIC was defined as the lowest concentration of LCFS at which a change in color from blue (oxidized resazurin) to pink (reduced resazurin) was not observed, indicating inhibition of bacterial growth.
The MBC was determined for extracts with noteworthy MIC values by inoculating the culture onto Brucella agar containing 7% horse blood plates. After incubation in microaerophilic conditions at 37 °C for 72 h, the colonies formed were subsequently computed. The MBC was defined as the lowest concentrations of the LCFS, inducing complete inhibition of colony formation of the test bacteria at the latter cultivation.
SEM
The effects of LCFS of L. fermentum T0701 inhibit H. pylori ATCC43504 on morphology of H. pylori cells were determined using SEM according to Kim et al.38 Briefly, H. pylori ATCC 43504 was cultivated MHB at 37 °C for 48 h. The bacterial suspensions were adjusted to 2 McFarland in a centrifuge tube containing 4X MIC of LCFS, incubated at 37 °C for 24 h under microaerophilic conditions. MRS broth was used as a negative control. After that the samples were then centrifuged at 10,000 rpm for 5 min and washes with phosphate-buffered saline (PBS) and centrifuged. Both control and LCFS-treated cells were dropped on a sterile glass coverslip (0.5 cm × 0.5 cm), and air dried. All samples were fixed using 2.5% (v/v) glutaraldehyde (Sigma-Aldrich, St Louis, MO, USA) in 0.1 M phosphate buffer for 24 h at 4 °C. Then, the cells were dehydrated in a graded ethanol series (40%, 60%, 80%, and 95% v/v) for 15 min each session, followed by two dehydration steps in 100% ethanol (Thermo Fisher Scientific, NY, USA) for 15 min followed by gold coating. Morphology of the bacteria after treatment with the LCFS was observed by field emission scanning electron microscope (Oxford Instruments, Quanta, Japan).
Cell culture
AGS cells (Passage number 5), a human gastric adenocarcinoma epithelial cells, ATCC CR-1739 (were purchased from American type culture collection, USA. AGS cells were cultured in Ham's F12 (Gibco, Thermo Fisher Scientific) with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin solution (Gibco) at 37 °C in 5% CO2. The AGS cells were subcultured and plated at 90% confluency.
Cell viability assay
The MTT assays were performed to assess the effect of L. fermentum T0701 LCFS on the viability of AGS cells according to a previously established procedure12, with slightly modifications. Briefly, AGS cells were seeded onto 96-well microplates at a density of 5 × 104 cells/mL and incubated at 37 °C in a 5% CO2 incubator for the cytotoxicity assays. The cells were then treated with LCFS (10.00–0.625 mg/mL) and incubated at 37 °C for 24 h. After incubation, the supernatants were discarded and the cells were washed with PBS. A volume of 100 µL of MTT solution (Sigma-Aldrich; 0.5 mg/mL in PBS) was added to each well and incubated for 4 h in the dark after removing the treatment mixture from each well. The formazan crystals were dissolved by adding 200 µL of dimethylsulfoxide solution (Sigma-Aldrich). The optical density was measured at 570 nm using a microplate reader. The experiment was repeated three times. The percentage cell viability was calculated using the following equation:
where Atest is the absorbance of the cells incubated with the medium containing LCFS, and Acontrol is the absorbance of the cells alone.
Anti-adhesion of H. pylori to AGS cells by LCFS
This experiment was performed as described by Lim et al.39 with minor modifications. Briefly, AGS cells were seeded onto 24-well microplates at 1 × 105 cells/mL and incubated at 37 °C in a 5% CO2 until a confluent monolayer formed, which typically occurred within 48 h. LCFS (5 mg/mL) was resuspended in Ham's F12 medium supplemented with or without H. pylori (108 CFU/mL) and then incubated for 2 h under microaerophilic conditions with stirring (200 rpm). The suspension was then added to a cell culture plate and incubated for 4 h to allow H. pylori to adhere to the cell lines. To assess the adhesion ability of H. pylori, cells were washed three times with PBS and lysed with 0.25% trypsin–EDTA for 5 min. The lysates were serially diluted in PBS and plated onto BA-containing 7% horse blood plates at 37 °C under microaerophilic conditions for 72 h. The number of H. pylori cells adhering to AGS cells was calculated as follows:
where V0 is the initial viable count and V1 is the viable count adhered to the AGS cells after incubation.
Co-culture of LCFS and H. pylori with AGS cells
The method was performed according to the procedure reported by Romyasamit et al.8 with minor modifications. The cytopathic effects of H. pylori on AGS cells were evaluated. First, AGS cells were seeded onto 24-well microplates at 1 × 105 cells/mL and incubated at 37 °C in a 5% CO2 until a confluent monolayer formed. LCFS (5 mg/mL) were resuspended in Ham's F12 medium supplemented with or without H. pylori (108 CFU/mL) and then incubated for 2 h under microaerophilic conditions with stirring (200 rpm). The supernatant was added to each well. The plates were incubated for 24 h at 37 °C in 5% CO2. The cells were examined under an inverted microscope and a commercial holotomographic microscope (HT-2H, Tomocube Inc.) for morphological changes.
Immunofluorescence assay
AGS cells treated with or without the co-culture of LCFS and H. pylori were analyzed using confocal microscopy. Immunofluorescence assays were conducted as described previously8,40. Briefly, AGS cells were seeded in eight-well plates and incubated until they reached a confluent state. The supernatant was then removed, and each supernatant containing LCFS was added to the wells with H. pylori or Ham's F12 media (negative control) and incubated for 24 h at 37 °C in 5% CO2. Subsequently, AGS cells were fixed with 300 μL of 3.7% formaldehyde for 10 min. The samples were washed four times and permeabilized with 0.1% Triton X-100 for 10 min. Non-specific binding sites were blocked by treatment with 1% BSA for 15 min. The Phalloidin-Alexa-Fluor-488 probe (Invitrogen, USA) was used at a dilution of 1:40, which toward F-actin was added, and incubated for 1 h at 4 °C in darkness. The nuclei of AGS cells were stained with 300 nM of DAPI (Sigma Chemical Co.) and incubated for 20 min. Finally, the samples were washed and 50 μL of anti-fade mountants (Invitrogen) was added prior to visualization under a Super-Resolution Laser Scanning Confocal Microscope; SR-LSCM (ZEISS, Germany) using a 63×/1.4 oil objective.
DNA isolation and whole-genome sequencing
Probiotics were previously isolated from fermented palm sap13. A single colony of L. fermentum T0701 was cultivated in MRS broth (HiMedia) at 37 °C for 24 h under anaerobic conditions. Genomic DNA was purified and extracted using a DNeasy extraction kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. The purity of the DNA was estimated using a spectrophotometer by measuring the absorbance at 260 and 280 nm (A260/A280) and 1.5% agarose gel electrophoresis. Purified genomic DNA was sequenced using a with NextSeq™ 550 System (Illumina, Inc., San Diego, CA, USA). The quality of the reads and low-quality reads were removed using Trim Galore (Galaxy Version 0.6.3) with default parameters.
Genome assembly and annotation
A dataset of 150-bp paired-end reads totaling 1 Gb pair was obtained from the sequence provider. Subsequently, the sequence reads were assembled and annotated using the comprehensive BacSeq v1.0 pipeline41 for assembly42, annotation4...
Comments
Post a Comment