A challenging case of carbapenem resistant Klebsiella pneumoniae-related pyogenic liver abscess with capsular ... - BMC Infectious Diseases
In December 2021, a 50-year-old man with clinical presentation of fever and chills was referred from Tehran and was admitted to the post-transplant ward of Abu Ali Sina Hospital in Shiraz, as the most important referral center for patients with liver diseases in Iran. The patient's medical history included diabetes mellitus (DM) and liver transplantation due to liver cirrhosis in December 2016. Biochemical tests revealed a white blood cell (WBC) count of 6000/µL, fasting blood sugar (FBS) level of 175 mg/dL, blood urea nitrogen (BUN) of 15 mg/dL, creatinine (Cr) of 1.1 mg/dL, C-reactive protein (CRP) of 96 mg/L, estimated sedimentation rate (ESR) of 105 mm/h, alkaline phosphatase (ALP) of 543 U/L, aspartate transaminase (AST) of 27 U/L, alanine transaminase (ALT) of 33 U/L, direct bilirubin (D. bilirubin) of 0.9 mg/dL, total bilirubin (T. bilirubin) of 2.1 mg/dL, and hemoglobin (Hb) of 8.2 g/dL (Table 1). Computed tomography (CT) revealed a 70 × 70 × 70 mm abscess in the right lobe of the liver. The patient's critical condition due to diabetes mellitus, and liver transplantation with multiple immunosuppressive drugs, necessitated empirical antibiotic treatment, pending culture and antimicrobial susceptibility report. Therefore, imipenem was prescribed. Treatment was started by administering imipenem-cilastatin 500 mg intravenously (IV) every 12 h and percutaneous catheter drainage of the liver abscess. We cultured the pus sample on MacConkey agar and blood agar and incubated it under aerobic and anaerobic conditions at 37 °C. K. pneumoniae was isolated and identified as the only pathogen by standard biochemical tests [13]. The K. pneumoniae isolate was confirmed to be K. pneumoniae by polymerase chain reaction (PCR) and DNA sequencing of the 16 S rRNA gene [3]. Antimicrobial susceptibility of K. pneumoniae isolate was determined by the Kirby-Bauer disk diffusion method according to Clinical Laboratory Standards Institute (CLSI) guidelines [14]. A panel of 12 antimicrobial agents was used, including ampicillin (AMP), ceftriaxone (CRO), cefotaxime (CTX), ceftazidime (CAZ), cefepime (CPM), aztreonam (ATM), imipenem (IMI), meropenem (MEM), ertapenem (ERP), gentamicin (CN), amikacin (AK), and ciprofloxacin (CIP). The K. pneumoniae isolate (mucoviscous colonies) was sensitive to all tested antibiotics except ampicillin [K. pneumoniae is intrinsically resistant to ampicillin]. On the 14th day of hospitalization, based on the patient's request, he was discharged, while the patient was advised to continue the treatment with imipenem 500 mg IV every 12 h and follow-up of the pigtail inserted in the liver abscess is necessary. He was admitted to a hospital in Tehran and received antibiotics for three weeks and was discharged with partial recovery.
After two weeks, the patient had progressive constitutional symptoms and fever. Therefore, he was again referred to Abu Ali Sina Hospital and admitted to the post-transplant ward. In the subdiaphragmatic part of the right lobe of the liver, there was a large liver abscess and pigtail catheter drainage was performed. Biochemical tests also revealed a WBC count of 15,700/µL, BUN level of 40 mg/dL, Cr of 1.8 mg/dL, ALP of 1203 U/L, AST of 84 U/L, ALT of 119 U/L, D. bilirubin of 3.1 mg/dL, T. bilirubin of 5.39 mg/dL, and Hb of 13 g/dL (Table 1). Antibiotic treatment was started by administrating colistin 4.5 × 106 units IV every 12 h and amikacin 500 mg IV every 12 h. Meanwhile, we isolated and identified two species of K. pneumoniae and E. coli in the pus culture. Here, the phenotypic and genotypic characteristics of the K. pneumoniae isolate had changed. On blood agar, the colonies were transparent with a sticky consistency and adhered to the culture medium (sticky mucoviscous colonies) (Fig. 1.A). The isolate was resistant to all tested antibiotics and was identified as a multidrug-resistant (MDR) isolate [15]. The minimum inhibitory concentration (MIC) of imipenem for K. pneumoniae was determined to be 128 µg/mL, by broth microdilution method according to CLSI recommendations [14]. MIC test for colistin was also performed by broth microdilution method according to CLSI guidelines [14] and the isolate was sensitive to colistin (MIC < 2 µg/mL). In addition, production of extended-spectrum β-lactamase (ESBL) and carbapenemase was detected by the phenotypic confirmatory disc diffusion test (PCDDT) and the modified carbapenem inactivation method (mCIM) according to CLSI guidelines [14]. Meanwhile, the E. coli isolate was also resistant to all tested antibiotics and was identified as an MDR isolate [15]. In K. pneumoniae and E. coli isolates, virulence genes entB, ybtS and antibiotic resistance genes blaTEM, blaSHV, blaCTX−M, and blaOXA−48 were detected by PCR using the specific primers [3] (Table 2). K. pneumoniae isolates belonged to sequence type 54 (ST54) by MLST and were identified as a non-K1/K2 ST54 cKp strain. On the 11th and 18th days of hospitalization, K. pneumoniae was again isolated and identified along with E. coli in pus culture. On the 20th day, despite the poor condition of the patient, he was discharged with personal consent and insistence and stated that he will continue his hospitalization and treatment in one of the hospitals in Tehran. The patient was advised that it is necessary to continue treatment with colistin 4.5 × 106 units IV every 12 h and amikacin 500 mg IV every 12 h and to follow up the pigtail inserted into the liver abscess.
After 6 weeks, the patient was again referred with pneumocath and shingles and was admitted to the emergency department. The patient stated that he was hospitalized for SARS-CoV-2 infection two weeks ago. The primary diagnosis was pleural effusion, shingles, and liver abscess. On the first day, a CT scan of the chest revealed that all parts of both lung fields were clear without active pulmonary infiltrates or fibrocystic disease. The patient was alert, with stable vital signs, apyretic, vesicular skin lesions in the background of painful erythema (shingles) on the left side of the face and neck, clear lungs, and purulent discharge from the catheter exit site with a positive K. pneumoniae culture. The patient's treatment continued with the administration of colistin, meropenem 500 mg IV every 12 h, and acyclovir. On the sixth day of hospitalization, the patient was alert, apyretic, ulcerative lesions on the face and neck were healing, clear lungs, and a pyogenic liver abscess of 200 × 130 × 61 mm with a positive culture of K. pneumoniae. Colistin, imipenem, doxycycline 100 mg every 12 h, and acyclovir were prescribed. Laboratory data revealed a WBC count of 10,200/µL, BUN level of 24 mg/dL, Cr of 1.5 mg/dL, ALP of 1416 U/L, AST of 30 U/L, ALT of 36 U/L, D. bilirubin of 0.6 mg/dL, T. bilirubin of 1 mg/dL, Hb of 7.4 g/dL (Table 1), and subsequently blood transfusion was prescribed to increase blood Hb. On the ninth day, while the liver abscess was very large, surgical drainage was performed. On the 11th day, the patient was alert, with stable vital signs, apyretic, and clear lungs. Purulent secretions were less and K. pneumoniae was detected in culture. The patient was receiving colistin, imipenem, tigecycline 50 mg IV every 12 h, fluconazole (used to treat fungal infections) 100 mg every 12 h, and acyclovir. Laboratory data revealed a WBC count of 6400/µL, BUN level of 32 mg/dL, Cr of 1.1 mg/dL, ALP of 1692 U/L, AST of 14 U/L, ALT of 19 U/L, D. bilirubin of 0.7 mg/dL, T. bilirubin of 1.4 mg/dL, Hb of 9.5 g/dL. On the 18th day, the patient was alert, with stable vital signs, apyretic, clear lungs, and shingles lesions were improving. The secretions were brief and K. pneumoniae was detected in the culture. Laboratory data revealed a WBC count of 6100/µL, BUN level of 14 mg/dL, Cr of 1.5 mg/dL, ALP of 1628 U/L, AST of 25 U/L, ALT of 45 U/L, D. bilirubin of 0.5 mg/dL, T. bilirubin of 0.9 mg/dL, Hb of 7.3 g/dL, and subsequently blood transfusion was prescribed to increase blood Hb. The patient was receiving colistin, doxycycline, and amikacin. On the 30th day, the patient was alert, with stable vital signs, apyretic, and K. pneumoniae was detected in the culture of the abscess secretions and Enterococcus sp. in the culture of the wound. On the 42nd day, the patient was alert, with stable vital signs, apyretic, and the shingles lesions were dry and improving. K. pneumoniae was detected in culture of liver abscess secretions. The patient was receiving colistin, tigecycline, doxycycline, fluconazole, and linezolid 600 mg every 12 h. On the 47th day, laboratory data revealed a WBC count of 7300/µL, BUN level of 15 mg/dL, Cr of 0.9 mg/dL, CRP of 8 mg/L, ALP of 4353 U/L, AST of 101 U/L, ALT of 208 U/L, D. bilirubin of 6 mg/dL, T. bilirubin of 12 mg/dL, and Hb of 7 g/dL (Table 1). The patient was discharged by personal desire, while all the professors were of the opinion that the patient should remain hospitalized. On the 60th day, the patient died while the clinical findings indicated that PLA was complicated by sepsis.
In order to investigate capsular polysaccharide expression, biofilm formation, and the presence of efflux pumps before and after imipenem treatment, a K1 ST23 hypervirulent K. pneumoniae (hvKp) strain, previously associated with invasive cryptogenic PLA [3], was included in the present study. HvKp produces a hypercapsule known as the hypermucoviscous phenotype, which is detected by a positive string test [3] (Fig. 1.B). The ability to form biofilm in two isolates sensitive (mucoviscous colonies) and CRKP (sticky mucoviscous colonies) isolated from non-cryptogenic PLA and hvKp isolate (hypermucoviscous colonies) isolated from cryptogenic PLA was investigated according to the previously described microtiter plate method [16]. All three K. pneumoniae isolates were biofilm producers. HvKp and sensitive cKp were identified as strong biofilm producers (optical density (OD) was 3.465 and 0.77, respectively), whereas CRKP was identified as a moderate biofilm producer (OD was 0.181) (Table 2). Interestingly, the biofilm formation ability of the hvKp strain was 16-fold higher than that of the positive control strain and almost 5-fold higher than that of sensitive cKp isolate, indicating a higher biofilm formation ability of hvKp compared to cKp. The presence of the efflux pump as an antibiotic resistance mechanism was also investigated according to the previously described ethidium bromide-agar cartwheel method [17]. The efflux pump mechanism could not be detected in all three isolates. Quantitative real-time PCR revealed that the expression of capsule wzi gene was nearly the same in hypermucoviscous and sticky mucoviscous isolates (cycle threshold (CT) was 22.47 and 23.12, respectively) and was approximately 1.3-fold that of the mucoviscous isolate (CT was 29.87) (Fig. 2).
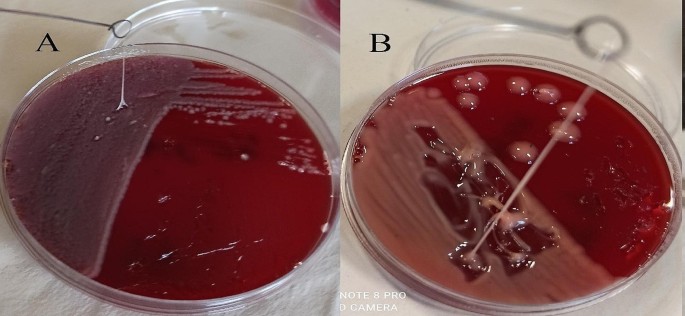
A. Sticky mucoviscous colonies of CRKP isolate. B. Hypermucoviscous colonies and positive string test of ST23 hvKp strain. A positive string test is defined as the formation of a viscous string > 5 mm in length when colonies grown overnight on a blood agar plate at 37 °C are stretched with a bacteriological loop
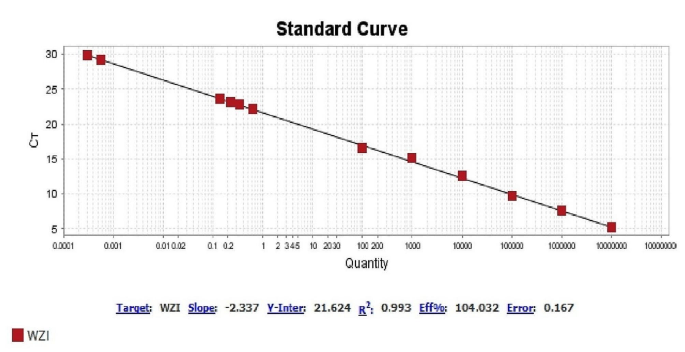
Expression of capsule wzi gene in two isolates sensitive (mucoviscous colonies) and CRKP (sticky mucoviscous colonies) and in hvKp strain (hypermucoviscous colonies)
Comments
Post a Comment