Strain-specific interspecies interactions between co-isolated pairs of Staphylococcus aureus and Pseudomonas aeruginosa from patients with tracheobronchitis or bronchial colonization | Scientific Reports - Nature.com
Abstract
Dual species interactions in co-isolated pairs of Staphylococcus aureus and Pseudomonas aeruginosa from patients with tracheobronchitis or bronchial colonization were examined. The genetic and phenotypic diversity between the isolates was high making the interactions detected strain-specific. Despite this, and the clinical origin of the strains, some interactions were common between some co-isolated pairs. For most pairs, P. aeruginosa exoproducts affected biofilm formation and reduced growth in vitro in its S. aureus counterpart. Conversely, S. aureus did not impair biofilm formation and stimulated swarming motility in P. aeruginosa. Co-culture in a medium that mimics respiratory mucus promoted coexistence and favored mixed microcolony formation within biofilms. Under these conditions, key genes controlled by quorum sensing were differentially regulated in both species in an isolate-dependent manner. Finally, co-infection in the acute infection model in Galleria mellonella larvae showed an additive effect only in the co-isolated pair in which P. aeruginosa affected less S. aureus growth. This work contributes to understanding the complex interspecies interactions between P. aeruginosa and S. aureus by studying strains isolated during acute infection.
Introduction
The concern about polymicrobial infections has been increasing, especially regarding the biofilm environment, where Staphylococcus aureus and Pseudomonas aeruginosa, members of the microbiota and potential pathogens, interact with each other1. Further, an important number of human infections are associated with biofilms development, especially those associated with indwelling medical devices such as orotracheal tubes and urinary catheters1,2. It has been postulated that the most common lifestyle adopted by bacteria in nature is to grow in biofilm mode where the microbial community protects itself from antimicrobial agents, including both drugs and host defenses. Indeed the biofilm environment favors the development of antimicrobial resistance through a variety of mechanisms3 and thus facilitates the emergence of multidrug resistant (MDR) microorganisms.
Staphylococcus aureus and P. aeruginosa are two of the major MDR nosocomial pathogens4 commonly associated with lower respiratory tract infections (LRTI) in diverse clinical settings5,6,7. Both microorganisms are among the most common in the pulmonary microbiota displaying a range of cooperative and competitive interactions, and their co-isolation has been observed in a wide range of airway diseases8. In biofilm-related infections, such as the ones occurring in cystic fibrosis (CF) or chronic wounds, in which S. aureus and P. aeruginosa have been co-isolated, disease outcomes have been seen more severe compared to single species infections1. Due to the intrinsic mechanisms of adaptation, survival and resistance to multiple classes of antibiotics9,10, their strain- and environment-specific interactions could play an important role in disease progression, the antimicrobial therapy choice and the clinical outcome11,12,13. While interactions between S. aureus and P. aeruginosa have been widely reported during the course of chronic pulmonary diseases, such as CF and chronic obstructive pulmonary disease (COPD)14, little has been investigated regarding these interactions in acute LRTI or bronchial colonization. Transition from occasional colonization into persistent respiratory infections could be facilitated, among many other factors, by the ability of pathogenic bacteria to form biofilms and tolerate antibiotic treatments13,15. In addition, the properties of S. aureus and P. aeruginosa strains associated with acute LRTI differ from those that chronically colonize the airways of CF patients16,17. The complex network of interactions between them involve changes in microbial behavior18,19 which in turn could enhance adaptation mechanisms or pathogenicity of microorganisms in the respiratory tract, such as the mixed-species biofilm formation20.
Both pathogens utilize cell–cell communication via different quorum sensing (QS) systems to coordinate the expression of multiple virulence factors and resistance determinants upon environmental stress conditions, as well as to compete with other members of the microbial community1,21. In S. aureus, the agr (accessory gene regulator) system is the most well studied QS network contributing to virulence in model biofilm-associated infections, although its exact role varies according to the type of infection model used22. Surfactant peptides secreted by S. aureus in a QS-controlled fashion have been associated with biofilm detachment and consequent bacterial dissemination23. On the other hand, among the variety of QS signaling molecules in P. aeruginosa, quinolone-based signals, with 2-heptyl-3-hydroxy-4-quilonone (PQS) as the main effector molecule, seem to induce the production of virulence factors in the presence of Gram-positive organisms, such as S. aureus 17,21,24. Two other QS systems in P. aeruginosa (LasR and RhIR) work regulating PQS through its main effector molecule, N-acylhomoserine lactone (AHL)1.
The study of the microbial synergies or interferences between S. aureus and P. aeruginosa in the context of acute LRTI or bronchial colonization could help to establish the real clinical role of these microorganisms in polymicrobial infections, as well as to gain further insight on how both pathogens interact with each other in the respiratory tract.
In this work, we investigated co-cultures and mixed biofilms formed in vitro by clinical strains of S. aureus and P. aeruginosa co-isolated from clinical respiratory samples after careful assessment on clinical diagnosis. We also determined the influence of their exoproducts and cross-interference in their QS system activities in planktonic and biofilm cultures using an artificial sputum medium. Finally, the interaction between both species was further assessed in the Galleria mellonella acute infection model. Differential genotypes and virulence phenotypes were observed among the strains studied here, and yet common traits were still detected in the interaction between each coexisting pairs of S. aureus and P. aeruginosa. This work also highlights the importance of working with clinical strains over other studies using laboratory strains.
Results
Inter-strain phenotypic diversity among the S. aureus and P. aeruginosa co-isolated pairs
Five co-isolated pairs of S. aureus and P. aeruginosa were obtained from five patients admitted to the ICU from Hospital Universitari Germans Trias i Pujol that were classified as having tracheobronchitis (3) and bronchial colonization (2) (Table 1). The clinical strains were first characterized based on species-specific virulence phenotypes (Supplementary Tables S1 and S2). In addition, genetic classification of the isolated S. aureus strains was done by DNA microarray. The five S. aureus strains belong to four clonal complexes and those strains with the same multilocus sequence typing (MLST) profile display different virulence genes as determined by DNA microarray. The agr genotyping also revealed the phenotypic diversity of the strains, and Agr was inferred to be functional in the isolates SAR2746, SAR7115 and SAR5091 as determined through δ-haemolysin detention in CAMP assay. Strains SAR7244 and SAR10471 were non-haemolytic on blood agar.
P. aeruginosa isolates also displayed significant diversity in terms of their genomic sequences, colony morphology and virulence related phenotypes. The MLST analysis shows that the five strains belong to different sequence types (STs) and the P. aeruginosa MLST database indicates a novel ST for strain PAR7244 (Table S2). Furthermore, none of the detected STs are part of the same clonal complex and none share more than two alleles in common. Virulence factors, such as swarming motility, fluorescent siderophore production, pyocyanin pigment and protease secretion were differentially expressed by all strains (Table S2 and Fig. S1).
Clinical isolates of P. aeruginosa induce slow-growing colonies in S. aureus
To investigate how the P. aeruginosa and S. aureus co-isolated pairs from patients with tracheobronchitis or bronchial colonization coexist and interact with each other, we first studied growth competition by co-culturing the two organisms in agar plates. The presence of P. aeruginosa inhibited growth of S. aureus on TSA plates as indicated by the halo formed around the bacterial colonies (Fig. 1A). Inside the inhibition halos, total killing was only observed in a narrow zone surrounding the P. aeruginosa colony except for strain SAR10471; beyond this area S. aureus colonies grew slowly and lost pigmentation. This effect caused by the diffusion of an inhibitory substance in a solid medium, could indicate the secretion of antistaphylococcal molecules by P. aeruginosa. Besides, the presence of P. aeruginosa supernatant in the medium also induced the formation of these small non-pigmented colonies, except for the isolated pair with ID 10471 (Fig. 1B and C). S. aureus SAR10471 was less affected if considering the inhibition halo diameter and no slow-growing colonies were observed in the presence of PAR10471 supernatant. The observed phenotypic changes in the other four S. aureus strains upon contact with P. aeruginosa were transient. For these isolates, the inhibitory effect of P. aeruginosa on S. aureus growth could be related to the production of phenazine pigments such as pyocyanin according to the culture medium used (Fig. S1). When the strain PAR10471 was grown on TSB, no pigment production was detected. Interestingly, this strain is the one that showed the lowest proteolytic activity (Table S2).
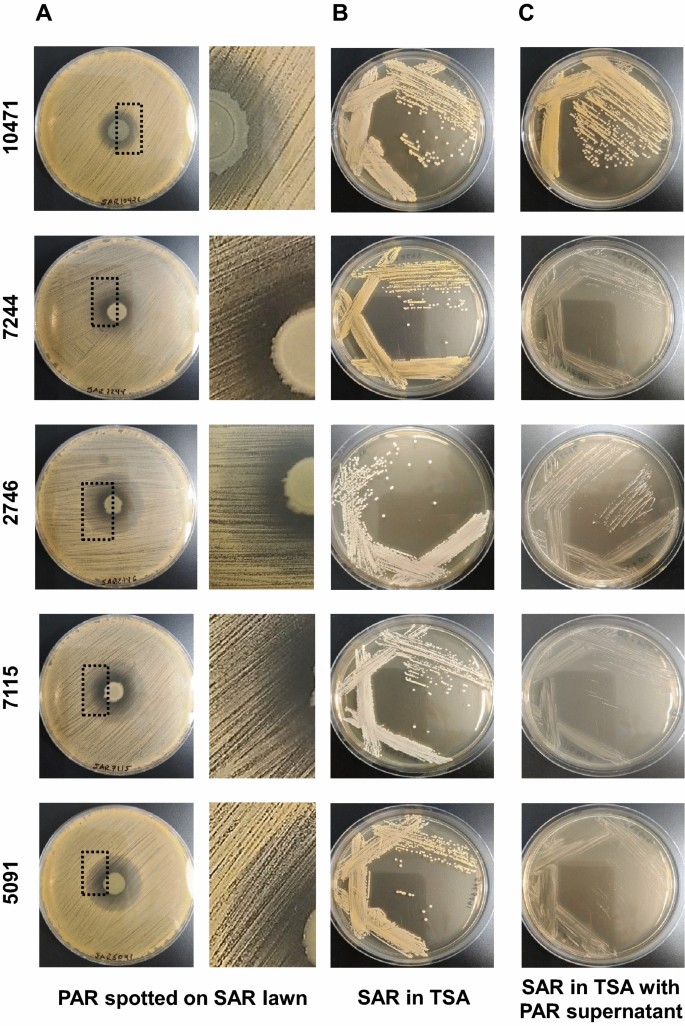
Interspecies growth competition assay on agar plates. (A) Colonies of P. aeruginosa (PAR) grown on a lawn of S. aureus (SAR) of the same co-isolated pair on tryptic soy agar (TSA) plates. Enlarged view of the boxed area is shown in the right panel. (B and C) S. aureus was streak-inoculated onto TSA plates with or without P. aeruginosa supernatant. Strain names are according to the origin of the sample (see Tables 1, S1 and S2).
Pseudomonas aeruginosa affect growth and biofilm formation in S. aureus, but show synergism in the early stages of biofilm formation in artificial sputum medium
To study competition between P. aeruginosa and S. aureus during biofilm formation, we first evaluated growth interference in planktonic conditions. For this purpose the two species were inoculated simultaneously in 0.5X TSB with 1% glucose and allowed to grow until the end of the mid-logarithmic phase. The numbers of viable cells collected from each species in both mixed and axenic (control) cultures are shown in Fig. 2A and B. For all pairs, except for the co-isolated pairs identified as 10471 and 7244, the presence of P. aeruginosa inhibits the growth of S. aureus in mixed cultures with more than tenfold reductions in viable cells. It must be noted that the strains of S. aureus SAR10471 and SAR7244 are genetically related and belong to the same clonal complex. In general, the growth of P. aeruginosa was not significantly affected in mixed cultures. In the competition assays in TSA plates, strains SAR10471 and SAR7244 were the least affected by their corresponding P. aeruginosa isolates if we take into account the diameter of the inhibition halos (Fig. 1).
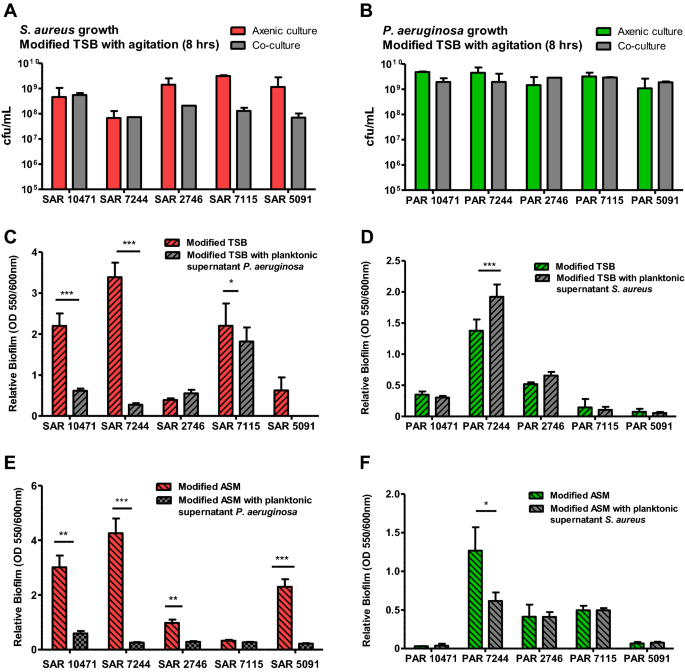
Effect of the interaction between clinical co-isolated pairs of S. aureus and P. aeruginosa on bacterial growth and biofilm formation. (A and B) Growth of S. aureus (SAR) and P. aeruginosa (PAR) strains in axenic culture and in co-culture with the corresponding co-isolated pair. The values represent the mean of viable bacterial count per ml (cfu/ml) after 8 h of cultivation at 37 °C with agitation. (C and E) Effect of the planktonic supernatants from P. aeruginosa on biofilm formation by the co-isolated S. aureus strain both in modified TSB or ASM medium at 37 °C. (D and F) Effect of the planktonic supernatants from S. aureus on biofilm formation by the co-isolated P. aeruginosa strain both in modified TSB or ASM medium at 37 °C. In all panels modified TSB consists of 0.5X TSB with 1% glucose, and modified ASM (artificial sputum medium) is a 1:1 mix of ASM (see methods) and 0.5X TSB with 1% glucose. When added, the planktonic supernatants are diluted 1:10 in culture medium. Error bars indicate standard deviation of biological replicates. *p < 0.05, **p < 0.01, ***p < 0.001 by Unpaired t test.
We also evaluated the effect of one species exoproducts on static biofilm formation on polystyrene plates by the other species using planktonic supernatants (Fig. 2C, D). Biofilm formation was tested in both 0.5X TSB supplemented with 1% glucose, a media that promotes robust biofilm growth for S. aureus in vitro25 and enhances their survival in the mixed biofilm26, and a TSB-based artificial sputum medium (ASM) to mimic growth in the cystic fibrosis lung habitat27. First, regarding the intrinsic capacity of each strain to form biofilm in both growth media (without the influence of the other species), a large phenotypic variability was observed between the strains of both species. For example, the S. aureus strains SAR2746 and SAR5091 with functional Agr form much less biofilm than the other ones. On the other hand, the P. aeruginosa strain PAR7244 is a strong biofilm former, while the strain PAR5091 almost does not form biofilm under the tested conditions. Despite this variability, and in almost all cases, the presence of P. aeruginosa supernatant significantly decreased biofilm formation by S. aureus under the two growth conditions used (Fig. 2C, E), with the exception of strain SAR2746 in TSB and strain SAR7115 in modified ASM. On the contrary, the presence of S. aureus supernatant did not affect biofilm formation by P. aeruginosa, except for the strain PAR7244 which showed variable results (Fig. 2D, F).
We studied mixed biofilm on polystyrene plates after 24 h of static growth in both TSB-based media (Fig. 3). Both strains were inoculated simultaneously at a ratio of 10:1 (S. aureus: P. aeruginosa) to prevent P. aeruginosa from totally outcompeting S. aureus. Under these conditions, and after 24 h of growth, the total amount of biofilm formed was lower compared to single-species biofilm (Fig. 3A). Even so, both species were collected from the formed biofilms but in different proportions (Fig. 3B). Irrespective of the co-isolated pair and the growth medium, the ratio of the two species in the biofilms after 24 h cultivation was lower than in the initial inoculum, with a higher proportion of P. aeruginosa. Differential cfu counting showed that the ASM-based medium increases this ratio in favor of S. aureus in the pairs 10471, 7244 and 5091 (Fig. 3B). The dual species biofilm in both media under static conditions on microscopy dishes were also visualized by confocal microscopy and representative images are presented in Figs. 3C and S2. When grown as a single species biofilm, S. aureus formed extensive patches of biomass after 24 h of growth, except for strains SAR10471 and SAR7115 that grew forming microcolony structures in artificial mucus medium (Fig. S2). On the contrary, all P. aeruginosa strains appeared as microcolonies in both growth conditions after 24 h of growth, except for strain PAR7244 that formed uniform sheets of biomass. This is probably because TSB is less capable of supporting P. aeruginosa biofilm formation under static conditions28, and also because this species takes up to 3 days to develop mature biofilm in ASM29,30. During co-culture, attachment of S. aureus cells to the surface of plastic dishes was negatively affected and the strains merely formed some localized cell aggregates. The initial formation of these microcolonies was favored in the modified ASM medium (Fig. 3C). In co-culture in the sputum-like medium, P. aeruginosa cells appear to have clustered around cellular aggregates formed by S. aureus, promoting the initial stage of mixed biofilm formation.
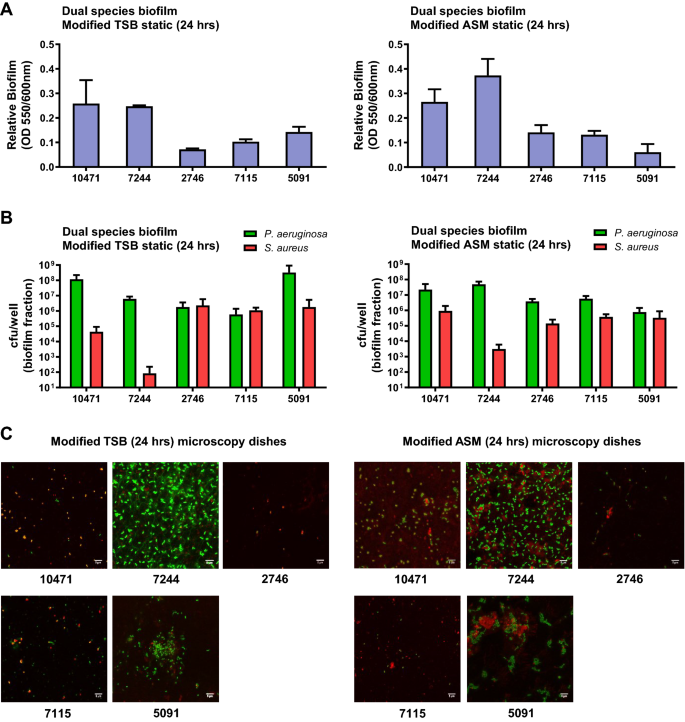
Attachment and microcolony formation in dual species static biofilms of clinical S. aureus and P. aeruginosa. Biofilm biomass measured by crystal violet method (A) and population of each bacterium in the mixed biofilm (B). Biofilms were grown under static conditions on polystyrene microtiter plates in 0.5X TSB supplemented with 1% glucose or in artificial sputum medium (ASM) mixed 1:1 with 0.5X TSB 1% glucose for 24 h. (C) Representative confocal laser scanning microscopy images of co-culture biofilms grown under static conditions on microscopy dishes for 24 h, and stained fluorescently for differentiation between species. Texas Red-X selectively binds to the surface of gram-positive bacteria to fluoresce red, while SYTOX counterstains the Gram negative fixed cells and fluoresces green. Scale bars represent 5 μm. In all experiments bacterial inoculation ratio was 10:1 (S. aureus:P. aeruginosa) and error bars indicate standard deviation of three biological replicates. Strain names are according to the origin of the sample (see Tables 1, S1 and S2).
Exposure to S. aureus supernatant influences swarming motility in P. aeruginosa
The effect of the presence of S. aureus strain, tested as culture supernatant, on swarming motility of the P. aeruginosa co-isolated strain was evaluated and the results are summarized in Fig. 4. In the three P. aeruginosa strains PAR5091, PAR7115 and PAR7244 exhibiting a dendritic swarm pattern, swarming motility increased in the presence of cell-free supernatants from its S. aureus counterpart. On the contrary, P. aeruginosa PAR10471 strain showed reduction on swarming motility in the presence of supernatant of the S. aureus co-isolated pair. Strain PAR2746 did not show motility under any of the conditions tested.
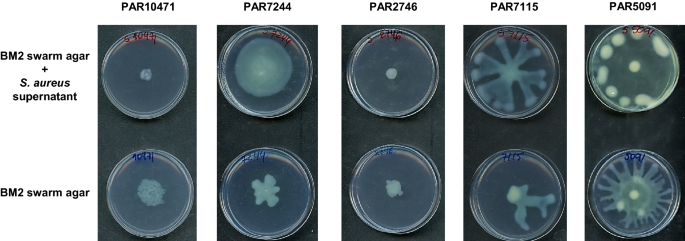
Effect of S. aureus supernatants on swarming motility of P. aeruginosa. Swarming motility was evaluated both in the absence and presence of the corresponding S. aureus supernatant diluted 1:10 in swarming medium (BM2 with 0.5% agar). Plates were incubated at 37 °C for 20 h. Images are representative of triplicate experiments.
Species specific quorum-sensing activities are differentially regulated in co-cultures
To evaluate the impact of the presence of one species on the quorum sensing activity of the other species of the co-isolated pair, we first studied the agr QS system of S. aureus. Expression of RNAIII, the effector of agr response, was measured by qRT-PCR in the five clinical strains grown under two conditions, in axenic cultures or in co-cultures with P. aeruginosa. ASM mixed 1:1 with 0.5X TSB supplemented with 1% glucose was used as the growth medium. When both species were grown together, the presence of P. aeruginosa affected the expression of the rnaIII gene compared with its expression in the axenic cultures in a strain-dependent manner (Fig. 5A). In the genetically related and Agr negative strains SAR10471 and SAR7244, rnaIII gene expression increased significantly (P < 0.001) in co-cultures. On the contrary, for the Agr positive strains SAR2746 and SAR7115 rnaIII expression decreased to levels that correlate with negative strains.
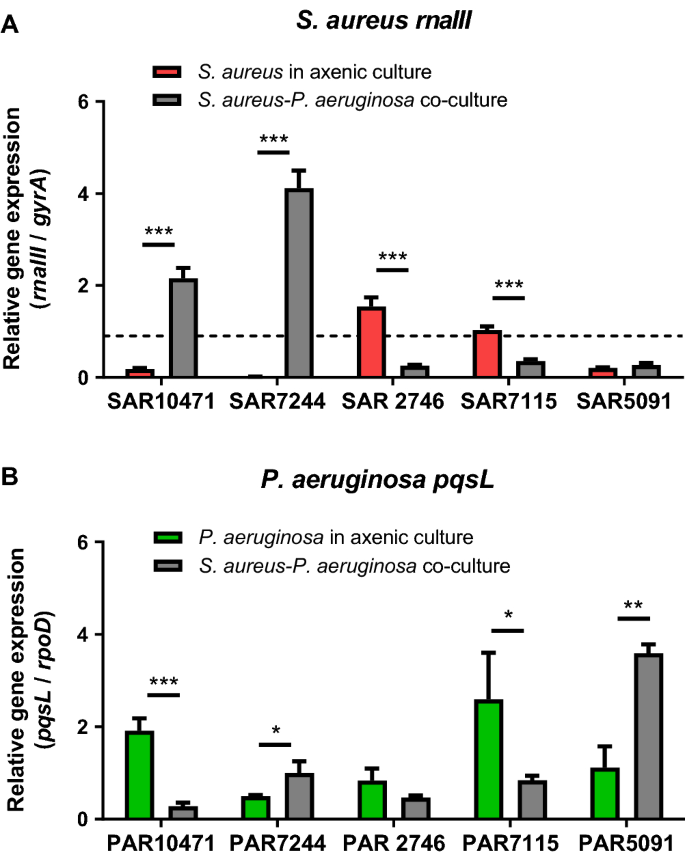
Differential deregulation of genes controlled by quorum sensing in co-culture. Relative expression of quorum sensing genes in co-cultures of S. aureus and P. aeruginosa in modified artificial sputum (ASM) medium (1:1 mix of ASM and 0.5X TSB with 1% glucose) assayed by qRT-PCR. (A) Effect of P. aeruginosa on rnaIII expression in S. aureus. Horizontal dashed line denotes the cut-off used to discriminate between Agr functional and dysfunctional strains (see methods). (B) Effect of S. aureus on pqsL expression in P. aeruginosa. In all experiments error bars indicate standard deviation of three biological replicates.
Using the same co-culture conditions in ASM modified medium, we studied the HQNO secretion system in P. aeruginosa strains by measuring expression of pqsL gene by qRT-PCR (Fig. 5B). PqsL is a mono-oxygenase that is required for the synthesis of the anti-staphylococcal compound HQNO which belongs to the PQS family31. In addition, expression of pqsL is suggested to play a role in PQS biosynthesis32. We had previously seen that the expression of pqsL is not detectable when the cells are grown in TSB medium; however, ASM medium positively regulated the expression of this gene in the P. aeruginosa strains. In co-culture with S. aureus pqsL expression was significantly affected, and once again it was in a strain dependent manner (Fig. 5B). The expression of pqsL significantly increased for the P. aeruginosa strains PAR5091 (P < 0.01) and PAR7244 (P < 0.05) in co-culture, the strains that showed the greatest negative impact on the growth of S. aureus (see Fig. 2). On the other hand, for the other three strains, the pqsL levels decreased in co-cultures.
The AHL quorum sensing system was also studied in P. aeruginosa isolates. All strains tested produced detectable levels of AHL, being the most producer strain PAR7115 and the strains PAR10471 and PAR2746 the lowest producer ones (Fig. S3). All strains synthesized long chain 3-oxo-AHLs including C8 and C10. In general, the presence of exoproducts from S. aureus did not significantly affect the production of AHL molecules by P. aeruginosa strains (Fig. S3).
Virulence of S. aureus and P. aeruginosa strains alone or in co-infection in G. mellonella larvae
Acute infection of G. mellonella larvae with individual clinical strains of S. aureus (1–6 × 106 cfu/larva) resulted in larval survival between 80 and 95% at 72 h post-infection (Fig. 6) for strains 10471, 7115 and 7244. S. aureus strains 2746 and 5091 were more virulent with a survival rate of 13% and 7% respectively in the same time period. On the other hand, since G. mellonella is highly sensitive to P. aeruginosa injected into the hemolymph33, infections with only 15–100 cells per larva were done. Under these conditions, the P. aeruginosa strains 7115, 2746 and 5091 killed all larvae within 96 h (Fig. 6). However, mortality was lower with the strains 10471 and 7244. Co-infection experiments with P. aeruginosa and S. aureus co-isolated strains resulted in a significant decrease in survival (P < 0.01) only for the strain pair 10471 (Fig. 6). For the other co-isolated pairs, the mixed infection did not show significant differences in comparison to the corresponding single species inocula that showed the most virulence.
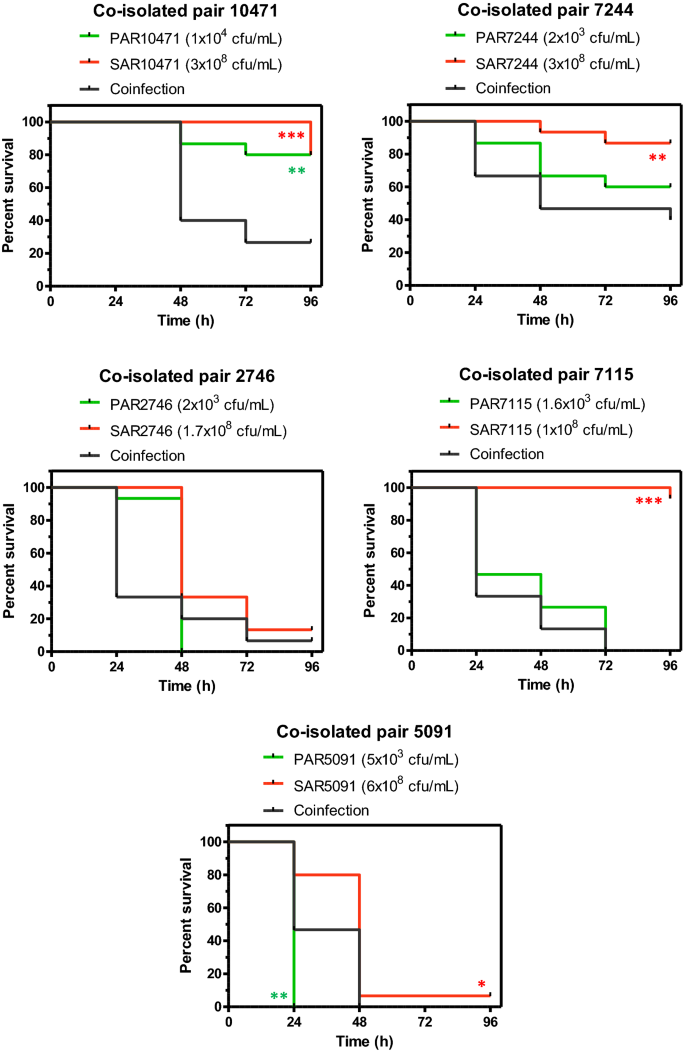
Co-infection in vivo shows an additive effect for the co-isolated pair with ID 10471. Survival of Galleria mellonella larvae over 96 h following infection with single species microorganisms (P. aeruginosa or S. aureus), or dual-species microorganisms (co-infection). Each panel represents a pair of co-isolated strains where each strain was used at the indicated infective dose prepared in PBS. Asterisks indicate significant differences relative to co-infected larvae, as assessed by the log-rank test. Only significant differences are shown.
Discussion
The interaction between clinical strains of P. aeruginosa and S. aureus has been extensively studied in recent years both in vivo and in vitro mostly from chronic infections1,8,21,34,35,36,37. Most of these studies have shown that when both bacteria are grown together, P. aeruginosa surpasses or decreases the S. aureus population. In the present work, this inhibitory effect of P. aeruginosa over its S. aureus counterpart has been observed for various clinical pairs co-isolated from the respiratory tract of patients with acute LRTI or bronchial colonization. However the strength of such an inhibitory effect has been strain specific. It has been shown that P. aeruginosa outcompetes S. aureus for the limited availability of required nutrients in the medium38. P. aeruginosa also produces an array of antimicrobial factors, which include respiratory inhibitors like hydrogen cyanide, pyocyanin, and the antistaphylococcal compound HQNO, as well as proteases that degrade wall components of S. aureus21,39,40. Particularly pyocyanin diverts electron flow and thus increases levels of products of oxygen reduction41. After lysis S. aureus could be an iron source for P. aeruginosa in cultures under iron-limiting conditions which contributes to the success of the latter39. Supplementary Fig. S4 summarizes the main interactions studied here between members of each co-isolated pair, and the phenotypic changes due to these interactions. Considering the high genetic variability of the clinical isolates and their clinical origin, it is not surprising that the interactions are strain-specific. Despite this, and the strong inhibitory effect exerted by P. aeruginosa on S. aureus under standard laboratory conditions, both species cooperate to survive in the mucosal environment of the respiratory tract.
The regulation of quorum sensing (QS) systems could play an important role in these dual species interactions. Secretion of anti-staphylococcal compounds is largely dependent on the modulation of the QS activity in P. aeruginosa in co-cultures. For example, HQNO is regulated by the quorum-sensing system PQS and it is involved in the increase of reactive oxygen species activity by disrupting the respiratory chain1,42. It has been shown that a P. aeruginosa mutant, deficient in the production of HQNO, does not efficiently kill S. aureus19. Exposure of S. aureus to HQNO has also been shown to down-regulate agr expression20. Beside this, several long-chain AHLs inhibit growth and virulence of S. aureus by reducing the agr activity43. All P. aeruginosa strains studied here synthesize long chain AHLs, and in general AHL production was not significantly affected by the presence of S. aureus exoproducts. However, co-culture with S. aureus seems to affect HQNO production in P. aeruginosa in a strain-specific manner. On the other hand, in S. aureus isolates with functional agr QS system, the exoproducts from co-isolated P. aeruginosa strains inhibit the expression of rnaIII, the regulatory effector molecule of this system. On the contrary, co-culture with P. aeruginosa activates rnaIII gene expression in strains with non-functional agr systems, which could also justify the specific strain pattern during the interaction between both species. QS network rewiring has been proposed as a strategy to coexist in the mucoid environment of the respiratory tract44. The observed increase in agr activity for strains SAR10471 and SAR7244 in co-cultures could correlate with a significant decrease in biofilm formation in the presence of P. aeruginosa supernatants (Fig. S4). Agr-mediated QS regulates many virulence factors in S. aureus that are important for the establishment of infection45,46. For instance, a functional Agr system is required for colonization in some infection models (e.g. epidermal), whereas reduced agr expression has been associated with increased adherence and biofilm production46,47.
Although this work is focused on strains isolated from patients with acute LRTI or bronchial colonization, the coexistence of P. aeruginosa and S. aureus can complicate both the course of the disease and the treatment decision making, promoting the transition into recalcitrant and chronic infections. For example, in CF patients carrying both microorganisms, co-infection is associated with worse disease outcomes and both pathogens seem to contribute additively to the disease severity1,48. Although as discussed before, P. aeruginosa impairs S. aureus growth, the co-occurrence of both microorganisms might also result in increased expression of some staphylococcal virulence factors49. Furthermore, S. aureus can survive in the presence of P. aeruginosa by the selection of tiny, non-pigmented colonies known as small-colony variants (SCVs). This phenotype is typically transient and it appears upon contact with exoproducts secreted by P. aeruginosa, such as pyocyanin and HQNO, that block the respiratory chain of S. aureus40,50,51. This phenotype can also be irreversible due to permanent genetic changes. S. aureus is able to persist in the airways of patients with CF by transforming into SCVs40, and this phenotype has been observed in biofilms after antibiotic therapies52. We have shown formation of slow-growing colonies of S. aureus by co-culturing on agar plates with P. aeruginosa, as demonstrated by others using CF patient co-isolates53. Regarding dual species biofilm formation, it has been shown that if S. aureus cells are able to survive killing by P. aeruginosa, certain extracellular factors of P. aeruginosa protect them within the biofilm matrix54,55. Interactions between S. aureus and P. aeruginosa could be reciprocally beneficial to each organism in terms of initial colonization and pathogenicity where co-aggregation plays a crucial role56. Here we also show that in co-culture, S. aureus cells start to attach and co-aggregate with P. aeruginosa on the plastic surface despite the strong inhibitory effect exerted by the latter. This synergistic effect is favored in conditions mimicking the CF pulmonary mucus.
On the other hand, the presence of molecules secreted by S. aureus increases P. aeruginosa virulence by enhancing the production of extracellular virulence factors such rhamnolipids, pyocyanin, elastase and quinolone-based signals1. A more recent study showed that S. aureus produces proteinaceous factors responsible for promoting P. aeruginosa surface motility57. In the present study exposure to S. aureus supernatant influenced swarming motility in the P. aeruginosa co-isolated strain, however this did not affect its ability to form biofilm. It has also been found by others that P. aeruginosa isolates from co-infected patients are less competitive with S. aureus36. Additionally, the presence of N-acetyl glucosamine from Gram-positive bacteria has been shown in vivo to increase the virulence of pathogenic P. aeruginosa in a G. mellonella infection model58. We have also detected this additive effect in the acute infection model in G. mellonella, but only for the co-isolated pair which showed the smallest competitive effect of P. aeruginosa on S. aureus growth (Fig. S4). Competition between S. aureus and P. aeruginosa has been also shown by others in the mouse model of acute lung infection53,59. In these studies it was also demonstrated that S. aureus profits from the presence of P. aeruginosa and the interactions between both species could be strain specific.
The processes and interactions governing biofilm development are directly involved in the pathogenesis of polymicrobial infections60. The functional and structural organization of both microorganisms in biofilms is complex and it is mainly regulated by their QS systems2. Within the biofilm, cells can be transformed phenotypically to become more resistant to treatments promoting the chronicity of infections. Dual species interactions within biofilms are dependent on both S. aureus and P. aeruginosa strains1,56, and this might explain the inconsistency between each co-isolated pair through all the experiments. Significant phenotypic differences were observed among the co-isolates studied here including differences in traits important for successful lung colonization. Considering the origin of the strains and their phenotypic diversity, our results add more clues on the complex interactions that take place between both species in the respiratory tract during acute LRTI or simply during a temporary colonization. Although strain-specific, these interactions could play an important role in disease progression and the clinical outcome in LRTI. This study also suggests the importance of the QS in dual species biofilm-related infections, making interference on these systems a promising therapeutic option. To deepen this knowledge, further studies should be conducted, especially how global gene expression and metabolome are affected in co-cultures.
Methods
Clinical isolates
Ten clinical isolates were included in the study. S. aureus and P. aeruginosa strains included were isolated simultaneously in the same respiratory sample (4 endotracheal aspirates and one sputum sample) (co-isolated) from five patients admitted to the ICU from Hospital Universitari Germans Trias i Pujol, a tertiary care hospital in Badalona (Spain) (Table 1). The patients were classified into respiratory study groups according to the Clinical Pulmonary Infection Score (CPIS) and as previously described61. At inclusion, all patients except one were under mechanical ventilation. Ethical approval was provided and the need for informed consent was waived by the Institutional Review Board: Comitè d'Ètica de la Investigació de l'Hospital Germans Trias i Pujol. Patient consent was waived because material used were strains and clinical data were anonymized. Sample collection followed the standard of care and was based on suspicion of infection.
Frozen glycerol stocks (15%) of the isolates were stored at -80˚C until use. For some experiments, the reference strains P. aeruginosa PAO1 and S. aureus RN6390B were included as controls. Unless otherwise stated, cultures were routinely grown in tryptic soy broth (TSB; BD Difco, Le Pont de Claire, France) at 37 °C and maintained on LB agar plates.
Mono-culture and co-culture planktonic growth
All growth curves experiments were done in 0.5X TSB supplemented with 1% glucose at 37 °C with agitation on a rotary shaker at 200 rpm. Overnight individual cultures of S. aureus and P. aeruginosa strains were diluted to an OD550 of 1.0 and used to inoculate (1:20) axenic or mixed cultures. For co-culture growth curves, bacteria were inoculated simultaneously at an equal ratio (1:1). Samples were collected every hour, serial diluted in PBS, and then seeded onto the selective media MacConkey agar (BD Difco) for P. aeruginosa and Mannitol salt agar (Conda, Madrid, Spain) for S. aureus. The agar plates were incubated at 37 °C for 24 h and the number of viable cells was determined.
Preparation of cell-free culture supernatant
Overnight cultures for each individual strain of both species were prepared in 100 mL of 0.5X TSB supplemented with 1% glucose for 18 h at 37 °C with shaking at 200 rpm. Planktonic supernatants were obtained after centrifugation at 4000 g for 20 min at 4 °C, followed by sterile filtration using a 0.22 µm-pore-size filter (Merck Millipore, Germany) and storage at − 20 °C until use. Before the use, supernatants were adjusted to pH 7.0, supplemented with 0.4% glucose and oxygenated by mixing.
Growth competition assay on agar plates
To prepare a bacterial lawn, overnight cultures of S. aureus were adjusted to OD550 of 0.01 and spread onto tryptic soy agar (TSA) plates using a sterile cotton swab. Five microliters of logarithmic cultures of P. aeruginosa isolates were spotted on the competitor lawns using a micropipette. In addition, fresh colonies of S. aureus were picked and streak-inoculated onto TSA plates with or without 10% (v/v) culture supernatant of P. aeruginosa grown in TSB. Plates were incubated 24 h at 37 °C.
Haemolysin activity in S. aureus
Hemolytic activities and Agr functionality of S. aureus were inferred using the classical CAMP synergistic haemolysis test on blood agar plates62. In CAMP assay, strains were tested against S. aureus strain RN4220 which produces only β-haemolysin. After incubation at 37 °C for 24 h, the plates were refrigerated to increase β-hemolytic activity.
DNA microarray-based genotyping of S. aureus
Genotypic characterization of S. aureus clinical isolates was performed with a species-specific genotyping DNA microarray (Alere Technologies GmbH, Jena, Germany), which detects specific resistance and virulence genes and their allelic variants and allows the assignment of isolates to MLST clonal complexes. PCR amplification and hybridization were performed following the manufacturer's instructions. An image of the array was recorded and analyzed using a designated reader and software (Arraymate, Iconoclust, Alere Technologies)63.
MLST typing of P. aeruginosa isolates
For each isolate, DNA was extracted using the DNeasy Ultra Clean Microbial ...
Comments
Post a Comment