How Salmonella grow together in the gut and exchange antibiotic resistance - Phys.org
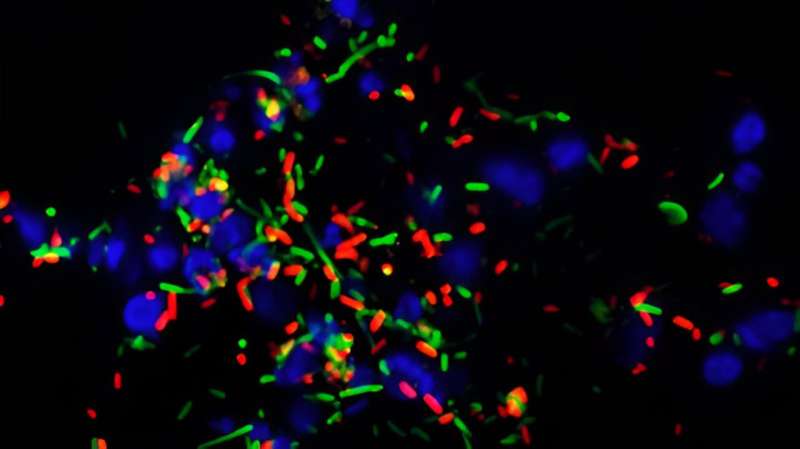
The ability to utilize a mere single alternative food source is all it takes for diarrhea-causing Salmonella bacteria to bloom when a gut is already colonized by a closely related strain, according to researchers from ETH Zurich. This coexistence enables the exchange of antibiotic resistance.
Bacteria are growing more resistance to common antibiotics, and one key factor contributing to this problem is the exchange of antibiotic resistance genes between closely related bacterial strains. When these related bacteria come into proximity, they can share information about how to survive antibiotics. Unfortunately, our intestines appear to provide an ideal environment for this exchange to occur. The reasons for this had remained unclear.
According to the classical theory, this exchange normally shouldn't happen at all, since microbial interactions prevent closely related bacteria strains to populate the same place at the same time.
The mammalian gut is home to thousands of bacterial species which interact closely with each other to form densely populated communities—the gut microbiota. These communities provide essential functions to their host, including resistance to pathogenic bacteria.
In the normal healthy gut, the resident microbiota prevents the colonization by enteric pathogens by various means, for example through competition for food molecules. And according to the niche exclusion theory, it is very challenging for bacteria of the same species to thrive in an already colonized gut, as they compete for the same food molecules.
A colonizing strategy of pathogen gut bacteria
"Recent observations seem to question this school of thinking, showing that several members of the Enterobacteriaceae family can co-exist in the gut," says Ersin Gül, a postdoc in the group of Wolf-Dietrich Hardt, Professor of Microbiology at ETH Zurich and member of the NCCR Microbiomes.
This presents a puzzling question for the researchers: how can closely related bacterial populations with similar nutrient needs coexist in the gut and even exchange information? Can this be predicted from the bacterial genomes?
To shed light on this puzzle, Hardt's team of researchers investigated the dynamics of bacterial coexistence in the gut. "We focused on understanding how a secondary group of bacteria can thrive when closely related resident bacteria are present," reports Gül, who is the first author of their study that specifically examined the behavior of pathogenic Salmonella bacteria, known for food-borne infections causing diarrhea, and related harmless E. coli strains. These results were recently published in the journal Cell Host & Microbe.
Diet matters
The researchers found that these bacteria utilize various food resources when they grow alone. In contrast, they can only grow and coexist with another Salmonella or E. coli population, if one population utilizes a type of food molecule, that the other one cannot consume (here, galactitol or arabinose).
This behavior reveals a metabolic strategy used by these bacteria to avoid competition and expand in a pre-occupied gut, and thereby enables the exchange of critical information between these microorganisms, including antibiotic survival mechanisms.
The findings underscore the profound impact of dietary components. Only if the right food molecules are there, pathogenic bacteria can co-infect our guts and exchange resistance to antibiotics. Through experiments involving mice, researchers revealed that the addition of a specific nutrient source enhanced the co-existence of two Salmonella populations and resulted in an increased number of antibiotic-resistant bacteria.
Consequently, our diet can unintentionally promote the dissemination of antibiotic resistance genes by providing nutrient sources that selectively favor the growth of particular bacterial populations.
New approaches against harmful bacterial invasion
The findings also have implications for the friendly bacteria in our guts since they might use the same colonization strategy. "Our study suggests that small differences in metabolic capacity at the species level may facilitate the replacement of existing microbiota strains with new ones," Gül explains.
Furthermore, these insights present exciting opportunities for much needed strategies to combat the spread of antibiotic resistance and promote a healthy gut microbiota. "Microbiota-based therapies may benefit from focusing on these alternative metabolic pathways," suggests Gül.
Hardt says, "The discovered principle is a bit like deciphering the hieroglyphs of the first sentence of the Rosetta stone—in the future, we might be able to systematically analyze bacterial genomes to predict if two strains can co-exist, exchange DNA and which foods might further promote this."
More information: Ersin Gül et al, Differences in carbon metabolic capacity fuel co-existence and plasmid transfer between Salmonella strains in the mouse gut, Cell Host & Microbe (2023). DOI: 10.1016/j.chom.2023.05.029
Provided by ETH Zurich
Citation: How Salmonella grow together in the gut and exchange antibiotic resistance (2023, August 21) retrieved 22 August 2023 from https://phys.org/news/2023-08-salmonella-gut-exchange-antibiotic-resistance.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
Comments
Post a Comment