Molecular investigations of Mycobacterium tuberculosis genotypes ... - BMC Infectious Diseases
Study population
Of the 390 potential participants that were investigated between November 2017 and January 2019 for the presence of M.tb, 26.41% (103/390) had positive TB disease on at least one occasion (Fig. 2) (Table 1).
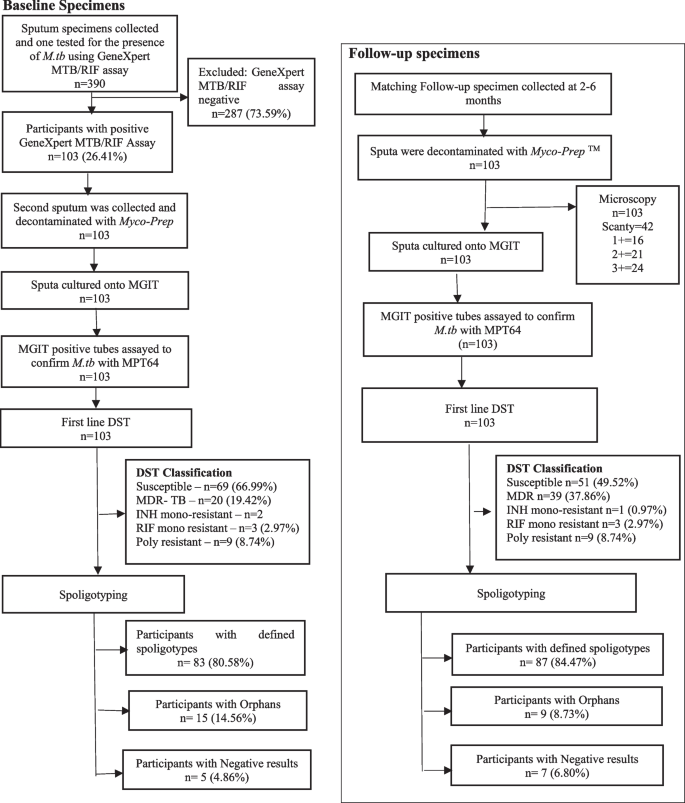
Participant flow diagram of baseline and follow-up specimens of patients collected at primary healthcare facilities in Eswatini. A total of n = 103 participants were enrolled into the study and followed through at 6th month
Drug susceptibility profile of the M.tb strains
The drug susceptibility testing (DST) results were generated at baseline (n = 103) and at follow-up, (n = 103). Although, most of the specimens at both baseline and follow-up (58.74%) were susceptible to all first-line anti-TB drugs, there was a significant proportion of specimens that were classified as RIF mono-resistant (28.16%). Furthermore, the poly-resistant specimens were 8.25% and those classified as INH mono-resistant were 4.85%. Notably, the region with a high proportion of MDR-TB specimens compared to the rest of the regions, was Lubombo, with 38.46%. Upon the analysis of the susceptibility patterns of the anti-TB drugs in all collected specimens (n = 206), it was revealed that most specimens had increased resistance to all the drugs at follow-up compared to baseline. Resistant specimens to RIF had significantly increased from 30.1% to 48.5% at follow-up (p = 0.005), with resistance to STR significantly increased from 27.2% to 39.8% (p = 0.038) and to EMB significantly increased from 21.4% to 36.9% (p = 0.011). Resistance to INH increased from 22.3% to 32.0%, however with no statistical significance (p = 0.079) (Table 2).
The drug susceptibility profiles were further analysed per region to determine the participant profiles among the different regions. Although most of the specimens in all the regions were susceptible to the four anti-TB drugs (Table 2), the Lubombo region had the highest drug resistance rates with all the anti-TB drugs tested. The resistance to RIF was 50% (p = 0.557), to INH was 42.3% (p = 0.197), to STR was 38.5% (p = 0.853) and to EMB was 34.6% (p = 0.639).
Notably, the analysed data suggests that a high proportion of participants infected with MDR-TB genotypes were from the Lubombo region. In addition, majority of these specimens in this region were resistant to all the anti-TB drugs.
The distribution of the TB phylogenic lineages among the participants
A total of 206 M.tb DNA samples that were extracted from the TB culture positive specimens for spoligotyping (103 at baseline and 103 at follow-up). Spoligotyping data revealed that 82.52% (170/206) genotypes had distinct spoligotyping patterns: 20.59% (35/170) genotypes detected from the Hhohho region, 54.1% (92/170) from the Manzini region, the Lubombo and Shiselweni regions with 12.94% (22/170) and 12.35% (21/170) genotypes, respectively. Few specimens (n = 36/206) were labelled orphans as their spoligotype patterns were not found in the SITVIT2 database.
The analyzed spoligotyping patterns revealed a total of four lineages: Indo-Oceanic (lineage 1), East Asian (lineage 2), Central Asian (lineage 3), and Euro-American (lineage 4) and twenty genotypes (EAI6-BGD1, EAI1_SOM, MANU1, MANU2, BEIJING, CAS_DELHI, CAS_KILI, T1, T2, S, X2, X3, LAM1, LAM3, LAM4, LAM5, LAM9, LAM11-ZWE, LAM-RUS,and T-H37Rv) among the study participants (Table 3) (Fig. 3).
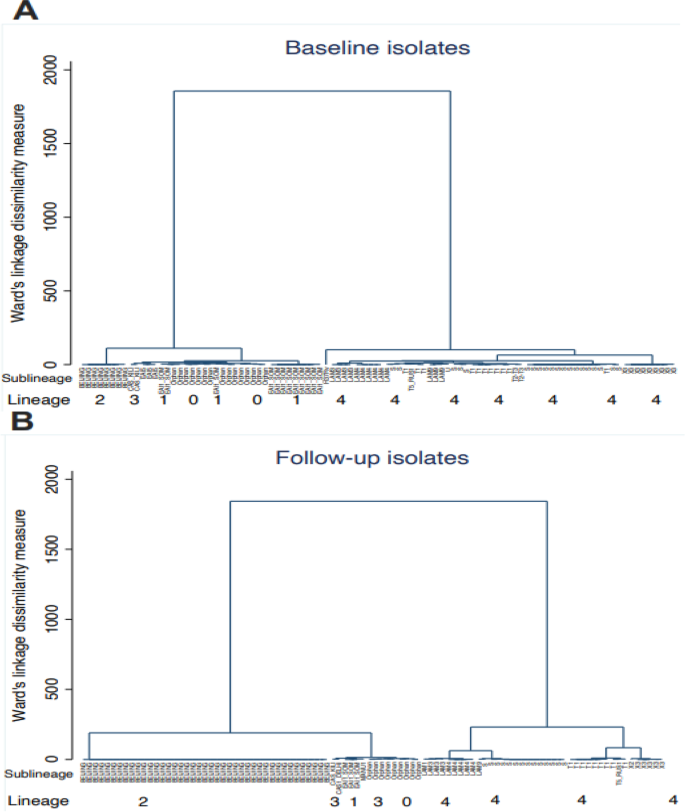
Spoligotype genotypes cluster analysis at baseline (diagram A) and follow up (diagram B). The figure shows the relationship between lineages and sublineages at baseline (diagram A) and follow-up (diagram B) to note clusters and measure of dissimilarity. Diagram (A) has 2 clusters, where the first cluster has lineage 1,3,1 and orphans and the second cluster is that of only lineage 4. The orphans are closely linked and similar to the lineage 1 EAI1_SOM and EAI5 clades. The second cluster of diagram (A) has only lineage 4 and simplicifolious in H37Rv sub lineage. The two clusters of diagram (A) have very small dissimilarities as the vertical heights are almost equal. The same observation on dissimilarity was made in diagram (B) which also two clusters. In the first cluster, lineage 2 has one clade compared to diagram (A) with two. The orphans are also linked to lineage 1 and 3 clades. The second cluster predominate sub lineage T1 has four clades which are close to X3 sub lineage. Comparing diagram (A) and (B), the measure of dissimilarity is higher in diagram (B)
The distribution of the lineages varied among the different regions. The Euro-American (Lineage 4) was the most common lineage with 46.12% (95/206) followed by the East Asian (Lineage 2) with 24.27% (50/206) and 9.71% (20/206) for Indo-Oceanic (Lineage 1) and 1.94% (4/206) for the Central Asian (Lineage 3). Furthermore, a high proportion of the Beijing genotype at 24.27% (50/206) and S genotype at 16.50% (34/206). The S genotype was predominant in the Shiselweni region with 30% at baseline and at follow-up (27.3%) in Lubombo region. Furthermore, the Beijing genotype was predominant at baseline in the Hhohho region with 23.5% and Shiselweni had more of the Beijing genotypes at follow up with 54.5%.
Notably, in most regions there was a diversity of genotypes between baseline and follow-up specimens (see Supplementary material and Table 3). Some of genotypes at baseline were not detected at follow-up timepoint. In the Hhohho region lineage 1 genotypes (EAI1_SOM and EAI6-BGD1) and CAS-Kili, LAM11-ZWE, LAM3 and LAM9 genotypes were only present at baseline and missing at follow-up. Also, the Manzini region had the CAS1-Kili, LAM3 LAM4, LAM9 and T2 genotypes were only noted at baseline. Likewise, in the Shiselweni region the EAI1_SOM, LAM4 and X2 were only present at baseline. Other regions had some genotypes that emerged at follow-up specimens. This was found in Hhohho region (LAM1, LAM4 and Manu2 genotypes),Manzini region (LAM-RUS genotypes) and Shiselweni region (LAM11-ZWE genotype) with some genotypes appearing in follow-up specimens. Interestingly, the frequency of the Beijing genotype, was observed to be highly present at follow-up specimens in most regions.
The Hhohho and Manzini regions had high diversity of TB genotypes, followed by the Lubombo region (n = 11/20) and Shiselweni region (n = 10/20). Some lineages had decreased frequency at follow-up compared to baseline. These were X3 and S genotypes in the Hhohho region; the LAM4 genotype in the Shiselweni region; EAI1_SOM, EAI6-BGD1 and S genotypes in the Manzini region. The same was noted in Lubombo region with the EAI1_SOM, EAI6-BGD1, X3, LAM3 and LAM-RUS genotypes. Moreover, some genotypes had the same frequencies between baseline and follow-up. The Beijing genotype was the most prevalent in follow-up specimens.
Further investigation reveled that some susceptible M.tb specimens later developed into MDR-TB and in the process acquired a completely new strain at follow-up timepoint. Comparison of both baseline (start of treatment) and follow-up genotypic data at six months or the last available positive specimen from patients on treatment was done. Our results showed that a significant proportion of follow-up specimens developed into MDR-TB (p = 0.0014) (Table 4) and Beijing was mostly detected in follow-up specimens (p < 0.000) (Table 5). Most of the Beijing genotypes were detected on third, fifth and sixth month (Fig. 4).
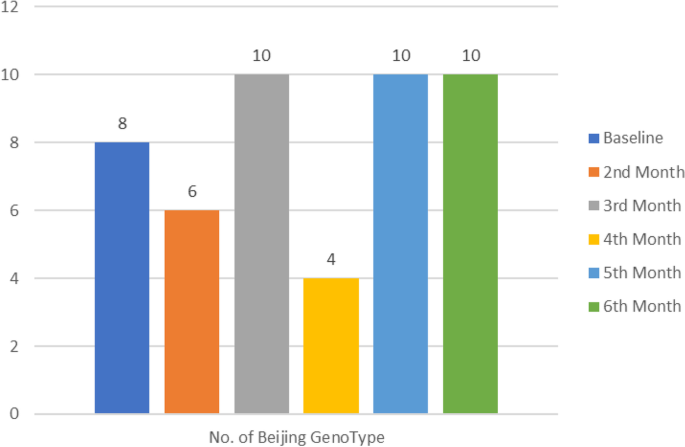
Detection of Beijing genotypes in serial isolates. At follow up, most Beijing strains were isolated on the 3rd, 5th and 6th month. The least were detected in the 4th month
Mixed-strain TB infection refers to TB disease caused by more than one clonally distinct M.tb strain, either through a single transmission event involving more than one distinct strain or through multiple transmission events (super-infection) during a single disease episode [12]. The study detected high number of isolates (64.5%; n = 133/206) that developed mixed-strain infections during treatment. The Beijing genotype had the highest number of mixed-strain infections, followed by S and T1 genotypes (Table 6). A total of 35 (23.3%) mixed-strain infections were resistant to all four first line drugs (Table 7).
Comments
Post a Comment