Global prevalence of asymptomatic norovirus infection in outbreaks: a systematic review and meta-analysis - BMC ... - BMC Infectious Diseases
Literature search and study characteristics
We identified 862 studies through an initial search. Of these, 491 were excluded because of repetition. The remaining 371 were reviewed using their titles and abstracts, and 302 that did not meet the inclusion criteria were excluded. Then we assessed the eligibility of the remaining 69 full-text articles. In this process, we screened the references cited by these articles, as well as the references for reviews and critical articles, adding a total of 23 articles. Of these 23 references, four, including one with mixed infections, were considered for the present study, while the remaining 19 references were either included in the 69 full-text articles or did not meet the inclusion criteria. Finally, 44 articles, 3 of which had mixed infections, were included for the analysis, with a total of 8,115 asymptomatic individuals. Because 10 studies reported outcomes in more than one subgroup, the number of studies in some particular subgroups used for the analysis would exceed the total number of studies included. Figure 1 is the flowchart of articles selection for this study. Table 1 shows the baseline characteristics and quality-assessment results of the studies included in the meta-analysis.
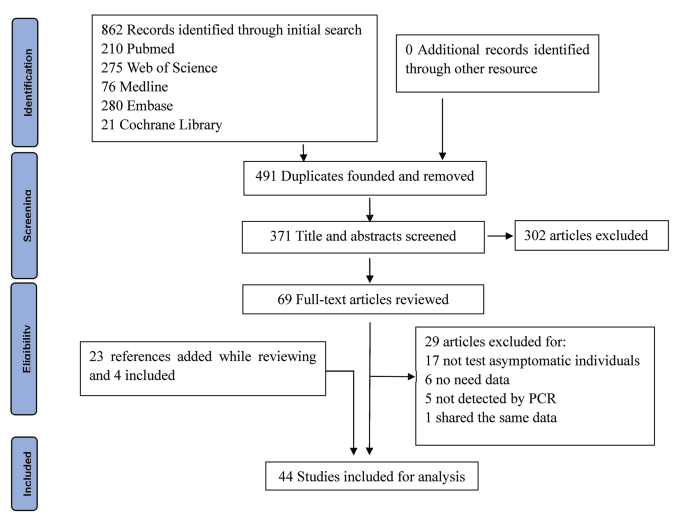
Flow diagram of studies selection
The 44 articles included in our study were from 13 countries; only two were from the Southern Hemisphere (Fig. 2). The maximum number of studies were from China (20), followed by Japan (6), Spain (4), United States (2), United Kingdom (2), the Netherlands (2), and Austria (2). Only one study was from other countries each such as Ireland, South Korea, Sweden, New Zealand, Italy and Brazil. The majority of the studies were conducted on adults (39). Four of these studies were on the whole population and only one specifically on the prevalence of non-gastrointestinal infectious diseases in children, although it did not mention their age. In addition, only one study was conducted on young children aged 9.2 ± 1.5 years. The outbreak settings comprised schools and other training institutions in 23 articles, catering places in 11 articles, medical institutions in 9 articles, and nursing homes in another 9 articles. Three articles did not report outbreaks settings in detail. In studies with NoV genotype information, 33.36% (472/1415) of positive asymptomatic individuals had GII, 0.92% (13/1415) had GI, 1.55% (22/1415) did not report the genotype (here, 1415 refers to the number of asymptomatic individuals with pathogens); and the rest (64.17%, 908/1415) reported genotypes, however, it was difficult to pinpoint the individual. In addition, 79.54% (35/44) of included articles reported only NoV GII, 2.27% (1/44) reported only NoV GI, 13.64% (6/44) reported both NoV GII and NoV GI, and 4.55% (2/44) reported no NoV genotypes. Among these studies, 37 clearly defined NoV cases or "asymptomatic" individuals, whereas seven studies mentioned "asymptomatic" but did not define them.
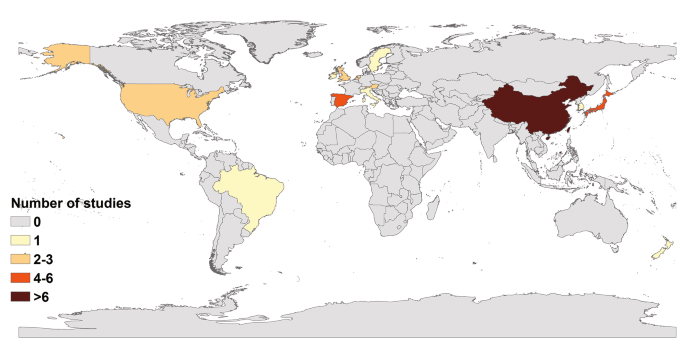
Studies distribution by countries
Meta-analysis of total asymptomatic prevalence
Prevalence of asymptomatic NoV infection in outbreaks was estimated to be 21.8% (95%CI, 17.4–27.3, I2 = 92%, τ2 = 0.4021, P < 0.01 test for heterogeneity) by using a random-effects model for the 44 articles included in this study (Fig. 3). Three studies on mixed infections were excluded then. The remaining 41 studies reported a prevalence rate of 21.8% for asymptomatic NoV infection in outbreaks (95%CI, 17.4–27.3, I2 = 93%, τ2 = 0.3990, P < 0.001 test for heterogeneity). No statistical difference was observed between the total prevalence of 41 and 44 studies. We performed a meta-regression analysis with a single covariate for the following 10 factors to determine the source of heterogeneity: geographical area, outbreak settings, outbreak seasons, sample types, genotypes, transmission routes, subjects' occupations, subjects' age, per capita national income, and presence or absence of a well-defined case and/or asymptomatic individual. Only the meta-regression analysis results for the geographical area were statistically significant (P = 0.012). The between-study variance decreased from 0.3369 to 0.2925, suggesting that it could explain 13.18% of the heterogeneity.
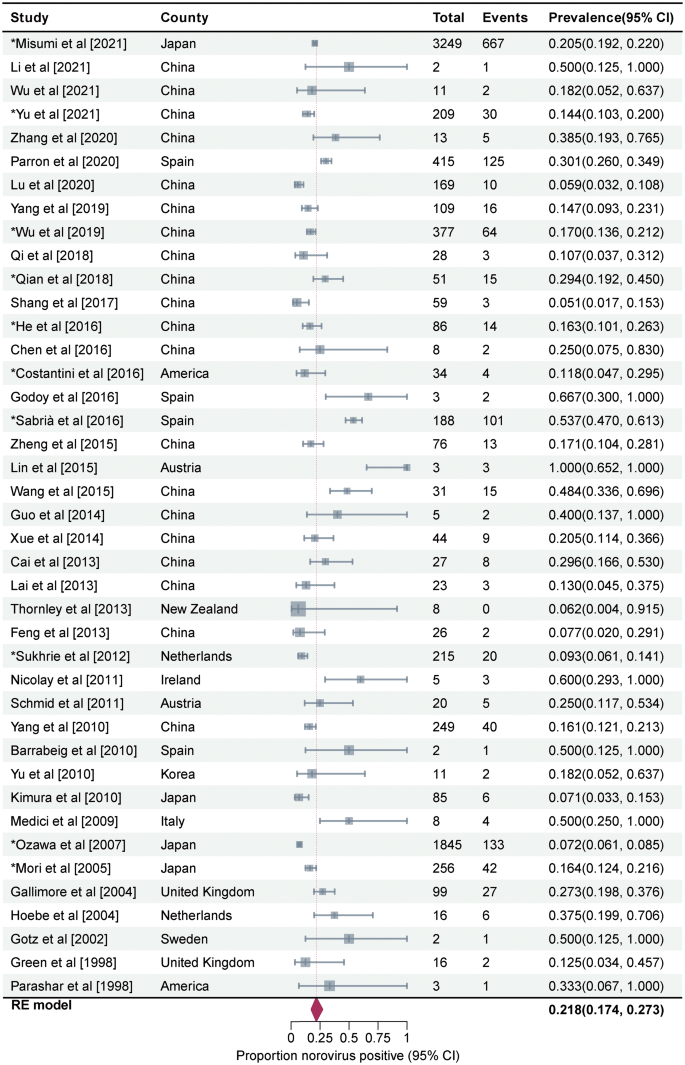
Forest graph. Results of 44 studies estimating the prevalence of asymptomatic NoV infection in the outbreaks (I2 = 92%, τ2 = 0.4021, P < 0.01 test for heterogeneity). Events: Number of NoV-positive asymptomatic individuals. Total: Number of asymptomatic individuals whose samples were detected. *Studies with prevalence were calculated in N outbreaks (N > 1)
Meta-analysis of subgroup asymptomatic prevalence
The pooled asymptomatic prevalence results for the subgroups are shown in Fig. 4. To determine the source of heterogeneity in subgroup analysis, it is necessary that the within-group heterogeneity should not be significant in any of the groups. However, the results of subgroup analysis showed that some groups had significant heterogeneity for each grouping factor (P < 0.05). That is, the source of heterogeneity could not be determined for the above 10 grouping factors.
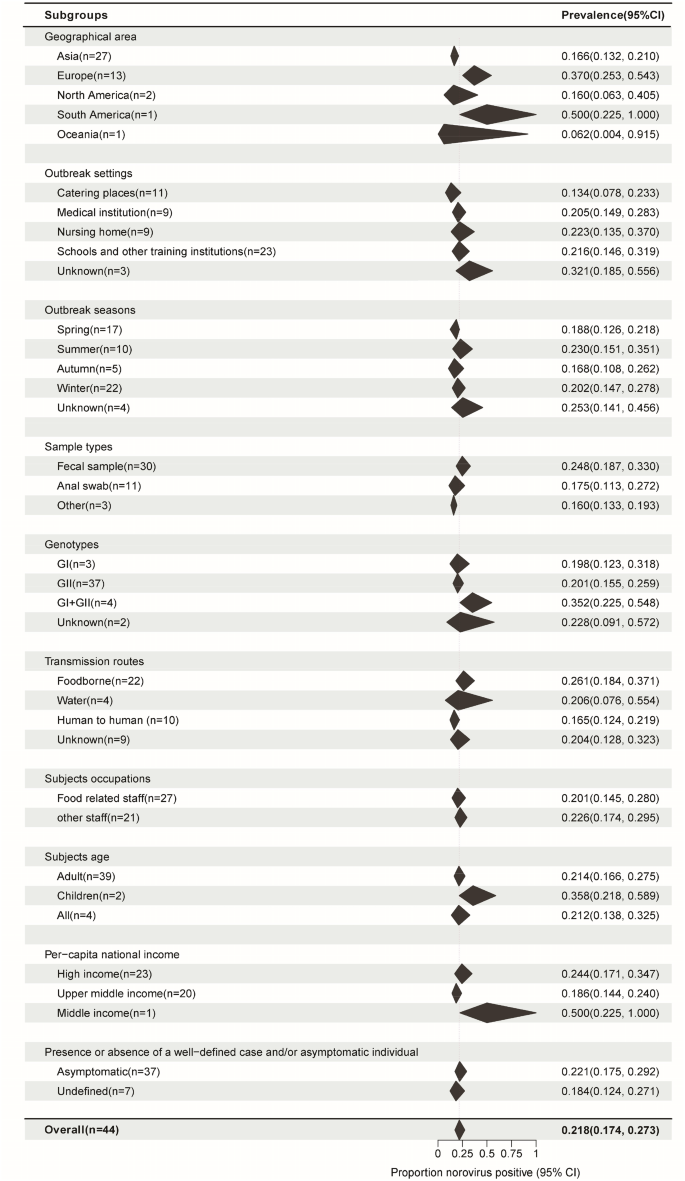
Subgroup pooled prevalence results. N: Number of studies. Black polygon: Estimated prevalence for each subgroup. Polygon width: Confidence interval of the pooled estimate. Because some studies involved information from multiple outbreaks, the number of studies from certain specific subgroups used in the analysis was not added to the total. Overall summary estimates were added to allow for comparison via the polygons with red dashed lines. For all subgroups, P values for heterogeneity tests P < 0.01. There are various sources of heterogeneity in prevalence studies. Subgroups divided by each factor still have significant heterogeneity
If we consider the geographic region, the prevalence was high in Europe (37.0%, 95%CI, 25.3–54.3) and low in East Asia (16.6%, 95%CI, 13.2–21.0) (P = 0.001). Among the outbreak settings, the prevalence rates in schools and other training institutions, medical institutions, nursing homes, and catering places were 21.6% (95%CI, 14.6–31.9), 20.5% (95%CI, 14.9–28.3), 22.3% (95%CI, 13.5–37.0), and 13.4% (95%CI, 7.8–23.3), respectively. However, the seasonal characteristics of the prevalence were not obvious; the highest prevalence was noted in the summer season (23.0%, 95%CI, 15.1–35.1), followed by that in the winter (20.2%, 95%CI, 14.7–27.8), spring (18.8%, 95%CI, 12.6–21.8), and autumn (16.8%, 95%CI, 10.8–26.2). The prevalence was the highest in foodborne outbreaks ((26.1%, 95%CI, 18.4–37.1), it was 25.0% (95%CI, 17.4–36.0) among food handlers (Fig. 5)), followed by waterborne outbreaks (20.6%, 95%CI, 7.6–55.4) and human-to-human outbreaks (16.5%, 95%CI, 12.4–21.9). Studies that collected only stool and anal swab samples reported prevalence rates of 24.8% (95%CI, 18.7–33.0) and 17.5% (95%CI, 11.3–27.2), respectively, while those that collected different specimens such as feces, vomitus, anal swabs, and throat swabs had a prevalence rate of 16.0% (95%CI, 13.3–19.3). The mixed infection prevalence of GI and GII NoV was the highest (35.2%, 95%CI, 22.5–54.8), followed by that of GII (20.1%, 95%CI, 15.5–25.9) and GI (19.8%, 95%CI, 12.3–31.8) alone. For NoV GII.4, the prevalence was estimated to be 19.6% (95%CI, 13.6–28.1) (Fig. 6), which was similar to that for other GII subtypes (18.2%, 95%CI, 12.4–26.8) (Supplementary Figure. S1) in outbreaks. The estimated prevalence for food handlers was 20.1% (95%CI, 14.5–28.0), which was similar to that for other occupational populations (22.6%, 95%CI, 17.4–29.5). The prevalence was higher in children (35.8%, 95%CI, 21.8–58.9) than it was in adults (21.4%, 95%CI, 16.6–27.5), as well as higher in high-income countries (24.4%, 95%CI, 17.1–34.7) than in upper-middle-income countries (18.6%, 95%CI, 14.4–24.0). The prevalence in studies that provided a clear definition of the case or asymptomatic individual was 22.1% (95%CI, 17.5–29.2), which was not significantly different from that in studies that did not provide such a clear definition (18.4%, 95%CI, 12.4–27.1). Except for the geographic region, the subgroup analysis of the other nine factors did not show any significant difference in prevalence among the groups.

Forest graph: Meta-analysis of 21 studies estimating the prevalence of asymptomatic food handlers in foodborne outbreaks (I2 = 79%, τ2 = 0.4500, P < 0.01 test for heterogeneity). Events: Number of NoV-positive asymptomatic individuals. Total: Number of asymptomatic individuals whose samples were detected. *Studies with prevalence were calculated in N outbreaks (N > 1)
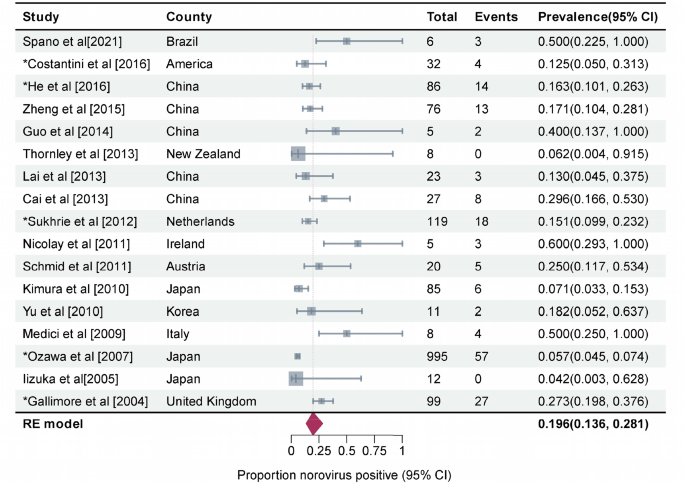
Forest graph: Meta-analysis of 17 studies estimating the prevalence of asymptomatic NoV GII.4 infection in outbreaks (I2 = 87%, τ2 = 0.3985, P < 0.01 test for heterogeneity). Events: Number of NoV-positive asymptomatic individuals. Total: Number of asymptomatic individuals whose samples were detected. *Studies with prevalence were calculated in N outbreaks (N > 1)
Sensitivity analysis and publication bias
Statistically, the funnel plot indicates that the scatter plots in the image are basically symmetric (Supplementary Figure. S2). According to the funnel plot and Peter's test results (P = 0.251), there was no significant publication bias. Sensitivity analysis was carried out by omitting one study at a time, and the results are very stable (Supplementary Figure. S3).
Comments
Post a Comment