Neonatal immune cells have heightened responses following in-utero exposure to chorioamnionitis or COVID-19 ... - Nature.com
Abstract
Background
Chorioamnionitis alters neonatal immune responses. Gestational COVID-19 infection is associated with adverse pregnancy outcomes, but its impact on neonatal immunity is unclear. We hypothesized that gestational COVID-19 exposure would result in exaggerated neonatal immune responses, similar to chorioamnionitis-exposed neonates.
Methods
Term umbilical cord blood mononuclear cells (CBMCs) were isolated from neonates exposed to chorioamnionitis, gestational COVID-19 or unexposed controls. CBMCs were cultured and stimulated with heat-killed Escherichia coli, Streptococcus agalactiae or Staphylococcus epidermidis. A multiplexed protein assay was used to measure cytokine levels in cell culture supernatants and flow cytometry was used to evaluate cellular-level cytokine expression.
Results
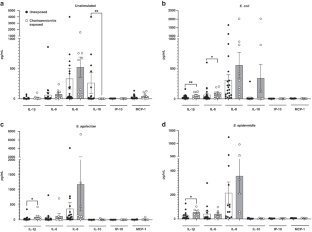
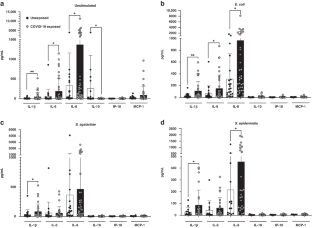
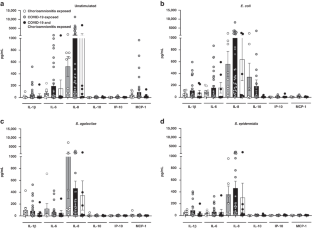
Both chorioamnionitis-exposed and COVID-19 exposed CBMCs demonstrated upregulation of IL-1β and IL-6 compared to unexposed CBMCs, while only COVID-19 exposure resulted in IL-8 upregulation. There were no differences between chorioamnionitis-exposed and COVID-19 exposed CBMCs when these groups were directly compared. Flow cytometry demonstrated immune cell subset specific differences in cytokine expression between the exposure groups.
Conclusion
The fetal/neonatal response to maternal inflammation differed based on immune cell subset and etiology of inflammation, but the global neonatal cytokine responses were similar between exposure groups. This suggests that targeting perinatal inflammation rather than the specific etiology may be a possible therapeutic approach.
Impact
-
Neonatal immune cells have similar pathogen-associated global cytokine responses, but different cell-level immune responses, following in-utero exposure to chorioamnionitis or COVID-19.
-
This is the first study to directly compare immune responses between neonates exposed to chorioamnionitis and COVID-19.
-
This suggests that the fetal/neonatal cellular response to perinatal inflammation differs based on the etiology and severity of maternal inflammation, but still results in a similar overall inflammatory profile regardless of the cause of perinatal inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Chorioamnionitis-exposure alters serum cytokine trends in premature neonates
A perfect storm: fetal inflammation and the developing immune system
Chorioamnionitis and neonatal outcomes
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Endlow, A. G., Li, J., Collier, A. Y., Atyeo, C. & Alter, G. Assessment of maternal and neonatal Sars-Cov-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the Covid-19 pandemic. JAMA Netw. Open 3, e2030455 (2020).
Liu, P. et al. The immunologic status of newborns born to Sars Cov2-infected mothers in Wuhan, China. J. Allergy Clin. Immunol. 146, 101–109 (2020).
Matute, J., Finander, B., Pepin, D., Ai, X. & Kalish, B. Single-cell immunophenotyping of the fetal immune response to maternal Sars-Cov-2 infection in late gestation. Pediatr. Res. 91, 1090–1090 (2022).
Neelam, V. et al. Pregnancy and infant outcomes by trimester of Sars-Cov-2 infection in pregnancy - Set-Net, 22 jurisdictions, January 25, 2020-December 31, 2020. Birth Defects Res. 115, 145–159 (2023).
Cosma, S. et al. Obstetric and neonatal outcomes after Sars-Cov-2 infection in the first trimester of pregnancy: a prosective comparative study. J. Obstet. Gynaecol. Res. 48, 393–401 (2022).
Foo, S. et al. The systemic inflammatory landscape of Covid-19 in pregnancy: extensive serum proteomic profiling of mother-infant Dyads with in Utero Sars-Cov-2. Cell Rep. Med. 16, 100453 (2021).
Zambrano, L. D. et al. Centers for disease control and prevention: Morbidity and Mortality Weekly Report (Mmwr). Weekly 69, 1641–1647 (2020).
Shanes, E. D. et al. Placental pathology in Covid-19. Am. J. Clin. Pathol. 154, 23–32 (2020).
Patberg, E. T. et al. Coronovirus disease 2019 infection and placental historpathology in women delivering at term. Am. J. Obstet. Gynecol. 224, 382.e381–382e388 (2020).
Romero, R., Chaemsaithong, P., Docheva, N., Korzeniewski, S. J. & Kim, Y. M. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J. Perinat. Med. 44, 53–76 (2016).
Committee Opinion No. 712. Intrapartum management of intraamniotic infection. Obstet. Gynecol. 130, e95–e101 (2017).
Rouse, D. J. et al. The maternal-fetal medicine units cesarean registry: chorioamnionitis at term and its duration–relationship to outcomes. Am. J. Obstet. Gynecol. 191, 211–216 (2004).
Sameshima, H., Ikenoue, T., Ikeda, T., Kamitomo, M. & Ibara, S. Association of nonreassuring fetal heart rate patterns and subsequent cerebral palsy in pregnancies with intrauterine bacterial infection. Am. J. Perinatol. 22, 181–187 (2005).
Malloy, M. H. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J. Perinatol. 34, 611–615 (2014).
Limperopoulos, C. et al. Positive screening for Autism in Ex preterm infants: prevalence and risk factors. Pediatrics 121, 758–765 (2008).
Bonnin, A. & Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 187, 1–7 (2011).
Goeden, N. et al. Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J. Neurosci. 36, 6041–6049 (2016).
Reuschel, E., Toelge, M., Entleutner, K., Deml, L. & Seelbach-Goebel, B. Cytokine profiles of umbilical cord blood mononuclear cells upon in vitro stimulation with lipopolysaccharides of different vaginal gram-negative bacteria. PLoS One 14, e0222465 (2019).
Bermick, J. et al. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J. 286, 82–109 (2019).
Stepanovich, G. et al. Chorioamnionitis-exposure alters serum cytokine trends in premature neonates. J. Perinatol. 43, 758–765 (2022).
Bergin, S. P., Thaden, J. T. & Ericson, J. E. Neonatal Escherichia Coli bloodstream infections: clinical outcomes and impact of initial antibiotics therapy. Pediatr. Infect. Dis. J. 34, 933–936 (2015).
Escobar, G. J. et al. Neonatal sepsis workups in infants >/=2000 grams at birth: a population-based study. Pediatrics 106, 256–263 (2000).
Nanduri, S. A. et al. Epidemiology of invasive early-onset and late-onset Group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 173, 224–233 (2019).
Chun-Chih, P., Jui-Hsing, C., Hsiang-Yu, L., Cheng, P. & Su, B. H. Intrauterine inflammation, infection or both (Triple I): a new concept for chorioamnionitis. Pediatr. Neonatol. 59, 231–237 (2018).
Berner, R., Csorba, J. & Brandis, M. Different cytokine expression in cord blood mononuclear cells after stimulation with neonatal sepsis or colonizing strains of Streptococcus Agalactiae. Pediatr. Res 49, 691–697 (2001).
de Jong, E., Hancock, D. G., Wells, C. & Currie, A. J. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylocccus Epidermidis. Immunol. Cell Biol. 96, 792–804 (2018).
Bermick, J., Watson, S., Lueschow, S. & McElroy, S. The fetal response to maternal inflammation is dependent upon maternal Il-6 in a Murine Model. Cytokine 167, e156210 (2023).
Ewald, J. T. et al. Inflammatory biomarker profiles in very preterm infants with the context of preeclampsia, chorioamnionitis, and clinically diagnosed postnatal infection. Pediatr. Rep. 15, 483–493 (2023).
Aghai, Z. H. et al. Ifn-G and Ip-10 in tracheal aspirates from premature infants: relationship with Bronchopulmonary Dysplasia. Pediatr. Pulmonol. 48, 8–13 (2013).
Rao, S. P. et al. Human peripheral blood mononuclear cells exhibit heterogeneous Cd52 expression levels and show differential sensitivity to Alemtuzumab mediated cytolysis. PLoS One 7, e39416 (2012).
Talla, A. et al. Persistent serum protein signatures define an inflammatory subcategory of long covid. Nat. Commun. 14, 3417 (2023).
Woodruff, M. C. et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long covid. Nat. Commun. 14, 4201 (2023).
Martin-Gonzalez, N. S. et al. Maternal respiratory viral infections during pregnancy and offspring's neurodevelopmental outcomes: a systematic review. Neurosci. Biobehav. Rev. 149, e105178 (2023).
Jung, E., Romero, R., Yeo, L., Chaur-Dong, H. & Para, R. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 25, e101146 (2020).
Jackson, C. M. et al. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr. Res. 81, 384–390 (2017).
Kamdar, S. et al. Perinatal inflammation influences but does not arrest rapid immune development in preterm babies. Nat. Commun. 11, 1284 (2020).
Garcia-Flores, V. et al. Maternal-fetal immune responses in pregnant women infected with Sars-Cov-2. Nat. Commun. 13, 320 (2022).
Gee, S. et al. The legacy of maternal Sars-Cov-2 infection on the immunology of the neonate. Nat. Immunol. 22, 1490–1502 (2021).
Bermick, J. & Schaller, M. Chorioamnionitis exposure dampens the preterm monocyte response to subsequent challenges. Immunol. Cell Biol. 96, 789–791 (2018).
Villamor-Martinez, E., Alvarez-Fuente, M., Ghazi, A. M., Degraeuwe, P. & Villamor, E. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw. Open 2, e1914611 (2019).
Getahun, D., Strickland, D., Zeiger, R. S., Fassett, M. & Jacobsen, S. J. Effect of chorioamnionitis on early childhood asthma. Arch. Pediatr. Adolesc. Med. 164, 187–192 (2010).
Smith, E. R. et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with Sars-Cov-2 infection: an individual participant data meta-analysis. BMJ Glob. Health 8, e009495 (2023).
Comments
Post a Comment