Oral and middle ear delivery of otitis media standard of care antibiotics, but not biofilm-targeted antibodies, alter ... - Nature.com
Abstract
Otitis media (OM) is one of the most globally pervasive pediatric conditions. Translocation of nasopharynx-resident opportunistic pathogens like nontypeable Haemophilus influenzae (NTHi) assimilates into polymicrobial middle ear biofilms, which promote OM pathogenesis and substantially diminish antibiotic efficacy. Oral or tympanostomy tube (TT)-delivered antibiotics remain the standard of care (SOC) despite consequences including secondary infection, dysbiosis, and antimicrobial resistance. Monoclonal antibodies (mAb) against two biofilm-associated structural proteins, NTHi-specific type IV pilus PilA (anti-rsPilA) and protective tip-region epitopes of NTHi integration host factor (anti-tip-chimer), were previously shown to disrupt biofilms and restore antibiotic sensitivity in vitro. However, the additional criterion for clinical relevance includes the absence of consequential microbiome alterations. Here, nine chinchilla cohorts (n = 3/cohort) without disease were established to evaluate whether TT delivery of mAbs disrupted nasopharyngeal or fecal microbiomes relative to SOC-OM antibiotics. Cohort treatments included a 7d regimen of oral amoxicillin-clavulanate (AC) or 2d regimen of TT-delivered mAb, AC, Trimethoprim-sulfamethoxazole (TS), ofloxacin, or saline. Fecal and nasopharyngeal lavage (NPL) samples were collected before and several days post treatment (DPT) for 16S sequencing. While antibiotic-treated cohorts displayed beta-diversity shifts (PERMANOVA, P < 0.05) and reductions in alpha diversity (q < 0.20) relative to baseline, mAb antibodies failed to affect diversity, indicating maintenance of a eubiotic state. Taxonomic and longitudinal analyses showed blooms in opportunistic pathogens (ANCOM) and greater magnitudes of compositional change (P < 0.05) following broad-spectrum antibiotic but not mAb treatments. Collectively, results showed broad-spectrum antibiotics induced significant fecal and nasopharyngeal microbiome disruption regardless of delivery route. Excitingly, biofilm-targeting antibodies had little effect on fecal and nasopharyngeal microbiomes.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Iron dysregulation and inflammatory stress erythropoiesis associates with long-term outcome of COVID-19

A novel antibiotic class targeting the lipopolysaccharide transporter
Introduction
Otitis media (OM), an inflammatory infection of the middle ear, is one of the most prevalent pediatric diseases worldwide with upwards of 80–90% of children under 5 years of age experiencing at least one episode1,2. The umbrella of OM encompasses a spectrum of clinical subtypes that include acute symptomatic OM (AOM), asymptomatic OM with effusion (OME), and chronic suppurative otitis media (CSOM) which are characterized by the presentation of symptoms, pathogenesis, and recurrence3,4. Surgical insertion of tympanostomy tubes (TT), a therapeutic intervention to permit middle ear ventilation while still permitting administration of topical antibiotics, can also introduce a risk of infection by translocated bacteria and subsequent post-tympanostomy tube otorrhea (PTTO) in over half of recipient children within a year of placement5. The global burden of OM is estimated to be 709 million AOM cases and 31 million CSOM cases with 51% and 22.5%, respectively, occurring in children under five6. Consequences of OM infections can include ear discomfort and fluid discharge, eardrum perforation, vestibular problems, and hearing impairment which exert negative influences on development, behavior, academic performance, and quality of life7. In the U.S. alone, the spectrum of OM clinical entities represents an annual healthcare burden of approximately $4 billion, not inclusive of indirect costs accrued by caregivers8,9.
Viral upper respiratory tract infections (URTI) by pathogens like respiratory syncytial virus, rhinovirus, influenza, and adenoviruses are among the most notorious predisposing factors for OM infections10,11,12. The collective sequelae of URTI—compromised airway defenses, negative middle ear pressure, and inflammation, obstruction, and dysfunction of the Eustachian tube—permit translocation of nasopharynx secretions and commensal bacteria into the sterile middle ear space13. Single-species or polymicrobial biofilms formed by aggregates of bacteria and adherent to middle ear mucosa both contribute to OM pathogenesis and confer substantial recalcitrance to antimicrobials as reviewed by Silva and Sillankorva14. Furthermore, the high cell density in biofilms has been shown to promote horizontal gene transfer of antimicrobial resistance (AMR)15. Despite mechanistic understanding of biofilm resistance to antimicrobials16,17 and the inability of antibiotics to reach concentrations capable of eradicating biofilms in the middle ear18, broad-spectrum antibiotics remain the standard of care in pediatric OM due to their short-term efficacy19. This practice unquestionably contributes to rapid, global emergence of antibiotic-resistant bacteria20, as well as microbiome dysbiosis to the potential detriment of immune development and overall health4.
Although the shift in microbiology is quite complex and not described in full here, certain selective pressures such as decades of pneumococcal conjugate vaccination (PCV) and widespread antibiotic use have played a role in shifting the microbiology of OM, once dominated by group A Streptococcus, to a predominant trio of nasopharynx-resident opportunistic otopathogens: Streptococcus pneumoniae, Moraxella catarrhalis, and nontypeable Haemophilus influenzae (NTHi)21. Currently, NTHi is attributed to half of all bacterial AOM cases and considered the primary cause of recurrent and chronic OM21. However, all three species have demonstrated coexistence in polymicrobial biofilms throughout OM disease progression22. More worrisome still, this trio of otopathogens exhibit concerning AMR profiles to antibiotics routinely used in OM treatment23,24,25. In addition to AMR and minimal efficacy of antibiotics on OM biofilm-resident bacteria, well-documented side effects of broad-spectrum antibiotics, including secondary infections, diarrhea, rash outbreaks, and disruption of the microbiome, present an added level of concern4,26. Per these considerations, demand for the development of alternative, sustainable strategies to combat OM pathogenesis and best minimize or eliminate these adverse outcomes are imperative.
While preventative strategies remain an ideal goal, therapeutic interventions for existing chronic or recurrent OM infections, such as CSOM or PTTO, are of equal importance. We have demonstrated that monoclonal antibodies (mAb) against two unique NTHi biofilm-associated determinants either induces NTHI to disperse from a biofilm or significantly disrupts biofilm structural elements to cause collapse of NTHi and polymicrobial biofilm matrices, both of which result in subsequent release of resident bacteria from their protective fortress27,28,29,30,31,32,33. Furthermore, these newly released (NRel) bacteria are phenotypically distinct from planktonic or biofilm-resident counterparts, susceptible to clearance by host immunity, and, importantly, demonstrate a four to eightfold increase in antibiotic sensitivity relative to planktonic states (free living)29,31. The first mAb, anti-rsPilA, targets the majority subunit of the NTHi Type IV pilus (PilA) which has critical roles in adherence, motility, competence, and biofilm formation34,35. The second mAb, anti-tip-chimer peptide, targets protective epitopes within the α- & β-tip domains of the bacterial DNA-binding DNABII protein integration host factor (IHF) produced by NTHi which encodes a critical biofilm structural element required for integration of extracellular DNA (eDNA) into biofilm matrices32. Both anti-rsPilA27,36 and anti-tip-chimer peptide30,36 have proven efficacious in inducing the NRel state and subsequent resolution of experimental NTHi-induced OM in vivo. We envision clinical application of these mAb technologies in biofilm-mediated infections, such as CSOM or PTTO, to facilitate eradication by host immune effectors and a now-efficacious shortened regimen of lower-dosage antibiotics if necessary. While targeted clearance of recalcitrant middle ear biofilms is the mechanistic crux of these potential therapeutics, an absence of significant microbiome dysbiosis is a critical prerequisite for success of these technologies in pediatric clinical settings. Moreover, anti-tip-chimer is derived from the DNABII protein family which has widespread roles in biofilm architecture37, therefore, potential collateral damage to commensal biofilm-forming bacteria associated with healthy microbiotas must be investigated.
We have previously shown that antibodies produced after subcutaneous and transcutaneous immunization with tip-chimer peptide do not alter the intestinal microbiome of chinchillas4. However, effects of clinically relevant TT delivery of anti-rsPilA and anti-tip-chimer mAbs on the microbiome have not yet been evaluated. To address this knowledge gap, nasopharyngeal lavage (NPL) and fecal samples were collected from chinchillas before and intermittently after antibiotic or mAb treatment to assess both nasal and gastrointestinal microbiome changes as fluids present in the middle ear can drain into the nasopharynx and subsequently the gut by way of the Eustachian tube and swallowing, respectively. Chinchillas were assigned to one of nine cohorts after TT insertion. One cohort received a week of twice daily oral amoxicillin-clavulanate (AC), reflective of standard OM care in children, and a positive control for antibiotic-mediated microbiome disruption. Alternatively and specifically toward the goal to develop a shorter-term yet more effective therapy for recalcitrant OM, other cohorts received a 2-day regimen of anti-rsPilA, anti-tip-chimer, AC, trimethoprim-sulfamethoxazole (TS), ofloxacin, or saline delivered via TT twice daily. For a summary of experimental design and analyses, see Fig. 1. As expected, oral AC treatment significantly impacted fecal diversity and differential abundance, in addition to unexpectedly analogous effects on NPL microbiomes. Shifts in the microbiome were also observed following TT delivery of AC, TS, and ofloxacin. Importantly, however, anti-rsPilA and anti-tip-chimer treatment did not significantly alter the microbiome, and instead, appeared similar to saline controls. These findings provide intriguing evidence of the effect of oral antibiotic administration on more proximal microbial environments like the nasopharynx, but more importantly, demonstrate that our anti-biofilm antibodies can be safely administered to healthy chinchillas without significant adverse effects on either fecal or nasopharyngeal microbiomes.
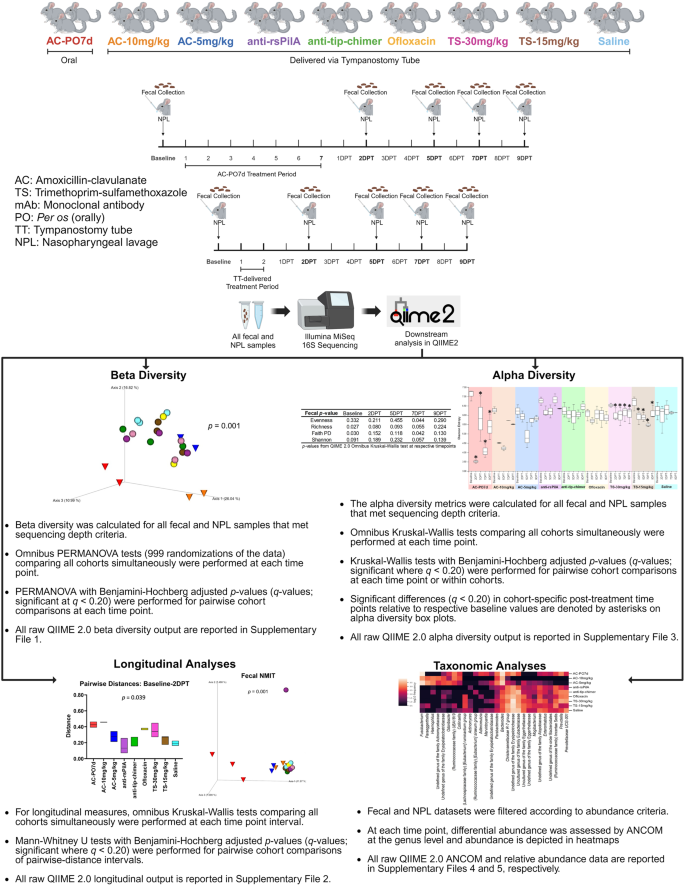
Representation of experimental design and subsequent microbiome analyses. Created with BioRender.com.
Results
16S rRNA gene sequencing
In total, 126 fecal and 126 NPL samples were available and submitted for 16S sequencing and returned a total of 2,448,804 high-quality sequences (19,435 ± 1303) and 1,505,762 high-quality sequences (11,951 ± 1131), respectively. After amplicon sequence variant (ASV) prevalence filtering and rarefaction, diversity analyses included 117 fecal samples comprised of 2,268,884 reads (19,393 ± 1192) and 1101 total ASVs. The 120 NPL samples comprised of 1,055,094 reads (8793 ± 698) and 453 total ASVs. Differential abundance analyses (without rarefaction) included 123 fecal samples comprising 1,938,547 reads (15,761 ± 944) and 382 total ASVs. The 124 NPL samples comprised of 836,105 reads (6743 ± 550) and 140 total ASVs. Cohort fecal and NPL sample sizes across time points are detailed in Tables 1 and 2, respectively.
Fecal beta diversity
Beta diversity, a measure of microbiome composition similarity or dissimilarity of two communities, was assessed using weighted UniFrac distances to compare all nine cohorts. Omnibus PERMANOVA tests were performed at each time point which compared all cohorts simultaneously to determine if there were differences between one or more of the nine cohorts, and P values from these tests are reported on respective principal coordinate analysis (PCoA) plots (Fig. 2). In addition, pairwise cohort comparisons were performed with permutational multivariate analysis of variance (PERMANOVA) and Benjamini–Hochberg correction for multiple testing to test for differences between each cohort relative to the others. Pairwise comparisons are reported as q-values (significant where q < 0.20) and are detailed in Supplementary File 1.
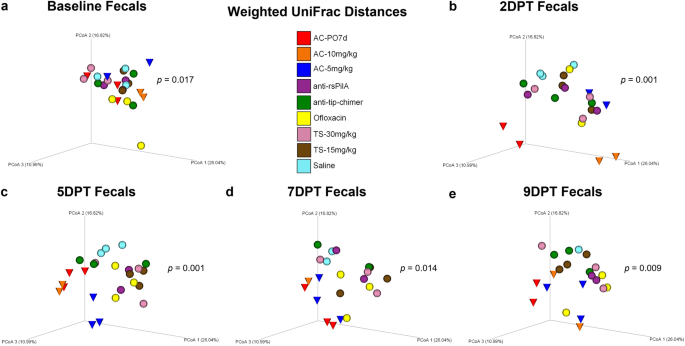
Principal coordinate analysis plots of weighted UniFrac distance matrices for fecal samples from all treatments at a baseline, b 2DPT, c 5DPT, d 7DPT, and e 9DPT. Differences in distances between treatments were analyzed in QIIME 2.0 by PERMANOVA with 999 randomizations of the data. Omnibus test PERMANOVA P values that tested for differences between the nine cohorts at each specified time point are displayed on respective graphs (significant where P < 0.05). Individual chinchillas are colored according to cohort treatment as depicted in the legend. Chinchillas in AC-treated cohorts are depicted as cones to emphasize separation from other cohorts which are depicted as spheres. Sample sizes are reported in Table 1.
Baseline weighted UniFrac distances revealed fecal microbial composition differed between cohorts (P = 0.017; Fig. 2a) prior to the administration of treatments. While unique microbial communities across individuals are not uncommon, conventional beta diversity metrics were supplemented with longitudinal analyses to account for intraindividual variability over time and more precisely delineate treatment-specific effects on the microbiome. Despite baseline differences, antibiotic effects on beta diversity became evident at posttreatment time points, and differences in fecal weighted UniFrac distances were noted at 2DPT (P = 0.001), 5DPT (P = 0.001), 7DPT (P = 0.014) and 9DPT (P = 0.009; Fig. 2b–e). As expected, an oral regimen of AC had dynamically disruptive effects on the gut microbiome per clear separation and clustering of AC-PO7d (red cones) away from TT-delivered treatments starting at 2DPT and persisting through 9DPT (Fig. 2). Separation and clustering of AC-10 mg/kg and AC-5 mg/kg (blue and orange cones, respectively) away from other TT-treated cohorts between 2DPT and 9DPT further delineated far-reaching impacts of broad-spectrum antibiotics and demonstrated that similar trends in microbiome disruption replicated whether AC was delivered orally or via TT (Fig. 2). Conversely, minimal changes in fecal beta diversity of mAb-treated cohorts (purple and green spheres) were observed across the experimental period which instead remained consistently clustered in the upper right quadrant of each weighted UniFrac PCoA. Pairwise comparisons of fecal weighted UniFrac distances between each cohort at posttreatment time points revealed AC-PO7d was significantly different from each of the eight TT-treated cohorts at 5DPT, including both TT-delivered AC cohorts, which conveyed the difference in magnitude of gut microbial disruption expected between oral and TT delivery of a broad-spectrum antibiotic (Benjamini–Hochberg adjusted P value of q < 0.20; Supplementary File 1). However, AC-10 mg/kg and AC-5 mg/kg were also different (q < 0.20) relative to all other TT-treated cohorts at 5DPT except anti-rsPilA. Importantly, neither mAb was significantly different from saline at any time point with the exception of 5DPT anti-rsPilA (q = 0.196; Supplementary File 1). Trends in posttreatment significance and separation of AC cohorts were similarly replicated in supplementary beta diversity metrics, Jaccard, Bray–Curtis, and unweighted UniFrac (Supplementary Figs. 1–3, respectively). Individual PCoA plots displaying cumulative changes across all time points for beta diversity metrics are available in Supplementary Fig. 4.
Fecal longitudinal beta diversity analyses
Sampling the microbiome of a single individual repeatedly over an experimental period, or longitudinally, provides valuable insight into overall microbiome stability as well as response and recovery from various treatments38. The longitudinal sample collection implemented in these studies, which consisted of a pre-treatment and four posttreatment time points, permitted a more thorough evaluation of the magnitude of change within an individual chinchilla's microbiome in response to the treatment received. In addition, longitudinal analyses were useful to elucidate treatment effects that may have been influenced or otherwise masked by baseline differences between cohorts. All longitudinal analyses were evaluated by omnibus Kruskal–Wallis tests comparing all cohorts simultaneously to determine if there were differences between one or more of the nine cohorts, and P values from these tests are reported on respective graphs or PCoA plots (Fig. 3). Pairwise cohort comparisons with Mann–Whitney U tests and Benjamini–Hochberg correction for multiple testing were used to test for differences between each cohort relative to the others. Longitudinal pairwise comparisons are reported as q-values (significant where q < 0.20) and are detailed in Supplementary File 2.
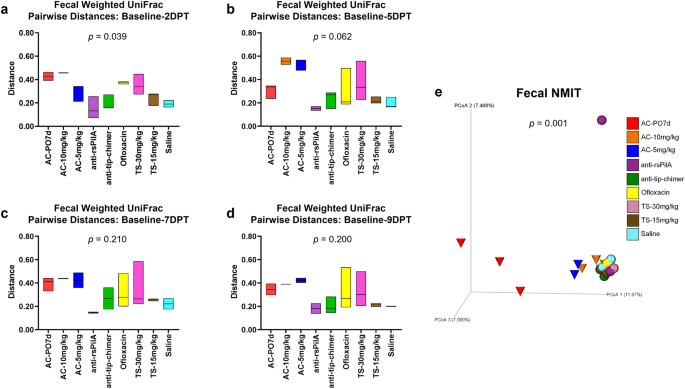
Longitudinal analysis of changes in fecal weighted UniFrac beta diversity pairwise-distances across posttreatment intervals a baseline—2DPT, b baseline—5DPT, c baseline—7DPT, and d baseline—9DPT. Omnibus tests for differences between any of the nine cohorts for each interval were performed with Kruskal–Wallis tests in QIIME 2.0 (P < 0.05) and P values for each interval are displayed on respective graphs. Floating bar charts denote minimum, maximum, and median values for pairwise-distances within cohorts between the respective time point intervals. Chinchillas without values for both time points in the assessed interval were not included in analysis. e Principal coordinate analysis plot of fecal nonparametric microbial interdependence test (NMIT) distances at the genus level for samples from all treatments. Individual cones (AC cohorts) or spheres (all other cohorts) represent the longitudinal change in microbial composition of each chinchilla (n = 27) across the experimental period. Differences in NMIT distances between treatments were analyzed in QIIME 2.0 by PERMANOVA with 999 randomizations of the data (P < 0.05) with P values displayed on respective graphs. Cohorts are colored according to treatment as denoted in the legend.
For each chinchilla, pairwise-distances, a measure of longitudinal changes in beta diversity distances in an individual, were calculated to assess the magnitude of change between paired samples (from the same chinchilla) collected at baseline and each posttreatment time point for a total of four pairwise-distance intervals (i.e., baseline—2DPT, baseline—5DPT, baseline—7DPT, baseline—9DPT). Intervals with greater deviations from zero represented greater amounts of change within a treatment relative to baseline values. Magnitudes of change across intervals, attributable to antibiotic treatment effects, could then be compared between cohorts. In accordance with fecal beta diversity at isolated time points, weighted UniFrac pairwise-distances revealed that the magnitude of change over the baseline—2DPT interval significantly differed across cohorts (P = 0.039). Expectedly, AC-PO7d and AC-10 mg/kg had numerically higher values over this interval, indicative of more prominent changes in the microbiome relative to other treatments. Interestingly, effects of ofloxacin and TS-30 mg/kg that were not as apparent in weighted UniFrac PCoAs also became evident. Most importantly however, both anti-rsPilA and anti-tip-chimer displayed some of the lowest values over the baseline—2DPT interval and were most similar to saline (Fig. 3a). A reduction in pairwise-distance values over the baseline—9DPT interval would suggest a return to baseline microbiome equilibrium if prior shifts had occurred, however, consistently high pairwise-distance values were maintained across all intervals in AC cohorts and again emphasized lasting effects of broad-spectrum antibiotic usage on the gut microbiota. In contrast, anti-rsPilA and anti-tip-chimer fecal pairwise-distances remained consistent, low, and comparable to saline across all intervals (Fig. 3a–d). No pairwise comparisons between cohort pairwise-distances were significant (Supplementary File 2).
A nonparametric microbial interdependence test (NMIT) was used to longitudinally compare similarity across individuals as a function of temporal microbial composition. Pairwise correlations of genus-level taxa composition within each individual over time were used to compute between-subject distances, and ultimately, generate a single compressed value representative of each chinchilla's longitudinal microbial interdependence. Similar clustering patterns suggest similar microbiome trajectories—or nature of change—over the experimental period, whereas distinct clustering suggests unique microbiome trajectories. Fecal NMIT definitively demonstrated that AC significantly altered microbial interdependence networks unique relative to TT-delivered antibiotics (P = 0.001) with especially remarkable separation of AC-PO7d from all cohorts. While oral AC effects were predictably more intense, lesser but still notable separation of both TT-delivered AC cohorts were similarly observed. The separation of the single anti-rsPilA (purple) sphere represents an individual chinchilla that showed signs of a suspected health problem (later confirmed) that led to its eventual removal from the study by 5DPT. Overall, non-AC cohorts, including mAbs, clustered tightly together and suggested a notably more minimal, or similar type, of overall change in the fecal microbiome (Fig. 3e).
Nasopharyngeal lavage beta diversity
Beta diversity in NPL samples was analyzed and is presented in a manner identical to that specified for fecal sample beta diversity analysis above. No differences in NPL microbiomes were observed at baseline. However, as in feces, NPL samples exhibited omnibus differences in 2DPT (P = 0.003) and 9DPT (P = 0.040) weighted UniFrac distances (Fig. 4b, e). Little change in temporal NPL clustering patterns were observed for any cohort except AC-PO7d and AC-10 mg/kg (red and orange cones, respectively) which demonstrated noticeably unique clustering at each time point (Fig. 4). Clustering of AC-5 mg/kg, which separated less distinctly than other AC cohorts, suggested a dose-dependent effect of TT-delivered AC on the nasopharyngeal microbiome. Although pairwise comparisons between cohorts did not significantly differ (Supplementary File 1), distinct clustering patterns and omnibus significance suggest that oral administration of AC disrupted the superiorly located nasopharyngeal microbiome and that all three AC treatments exerted prolonged effects on NPL beta diversity. Similar trends in significance were observed for supplemental beta diversity metrics, Jaccard and Bray–Curtis, but not unweighted UniFrac (Supplementary Figs. 5–7, respectively). Cumulative changes in NPL beta diversity metrics are provided in Supplementary Fig. 8. In general, weighted UniFrac changes appeared to be less extreme in nasopharyngeal microbiomes than observed in feces. More importantly, however, neither mAb-treated cohort exhibited notable shifts in NPL weighted UniFrac clustering over the experimental period, recapitulated fecal findings, and showed a lack of mAb-associated microbiome disruption.
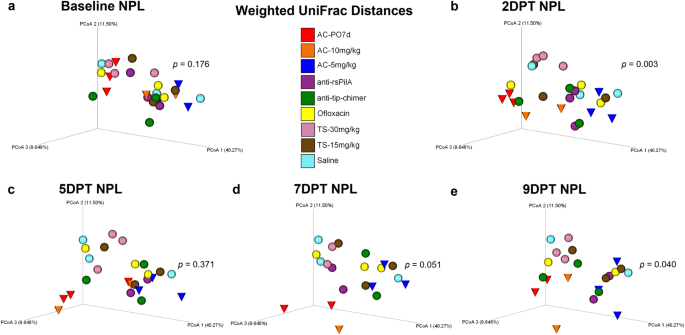
Principal coordinate analysis plots of weighted UniFrac distance matrices for NPL samples from all treatments at a baseline, b 2DPT, c 5DPT, d 7DPT, and e 9DPT. Differences in distances between treatments were analyzed in QIIME 2.0 by PERMANOVA with 999 randomizations of the data. Omnibus test PERMANOVA P values that tested for differences between the nine cohorts at each specified time point are displayed on respective graphs (significant where P < 0.05). Individual chinchillas are colored according to cohort treatment as depicted in the legend. Chinchillas in AC-treated cohorts are depicted as cones to emphasize separation from other cohorts which are depicted as spheres. Sample sizes are reported in Table 2.
Nasopharyngeal lavage longitudinal beta diversity analyses
Longitudinal analyses performed on NPL samples were analyzed and presented in a manner identical to that specified for longitudinal fecal sample analyses above. Weighted UniFrac pairwise-distances in NPL samples were numerically greater for AC-treated cohorts, similarl to fecal samples, but no differences were detected via omnibus tests or through pairwise comparisons between cohorts (Fig. 5a–d and Supplementary File 2, respectively). Overall, anti-rsPilA and anti-tip-chimer exhibited low and overall stable pairwise-distances across the intervals, indicating minimal changes from baseline. Longitudinal NMIT analysis of NPL samples (Fig. 5e) showed the same distinctly clustered nasopharyngeal microbiome trajectory for AC-PO7d as observed in fecal samples, but the separation of AC-10 mg/kg and AC-5 mg/kg was less apparent in the nasopharynx. The separation of a single anti-rsPilA purple sphere and AC-10 mg/kg orange cone represent individuals that had suspected health problems or were found as mortalities leading to removal from the study by 5DPT. Overall, AC was most disruptive to nasopharyngeal microbiomes, whereas mAbs did not lead to significant microbiome disruption relative to baseline compositions.
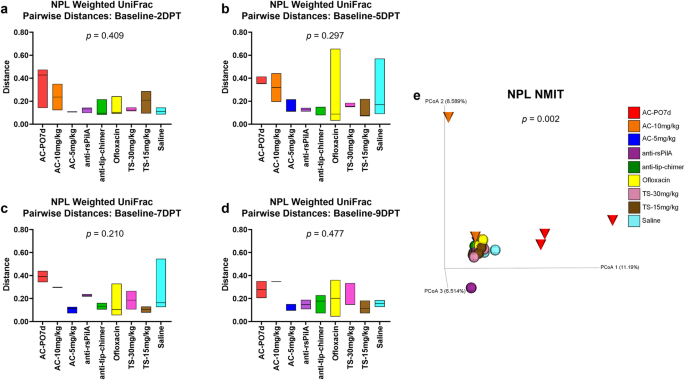
Longitudinal analysis of changes in NPL weighted UniFrac beta diversity pairwise-distances across posttreatment intervals a baseline—2DPT, b baseline—5DPT, c baseline—7DPT, and d baseline—9DPT. Omnibus tests for differences between any of the nine cohorts for each interval were performed with Kruskal–Wallis tests in QIIME 2.0 (P < 0.05) and P values for each interval are displayed on respective graphs. Floating bar charts denote minimum, maximum, and median values for pairwise-distances within cohorts between the respective time point intervals. Chinchillas without values for both time points in the assessed interval were not included in the analysis. e Principal coordinate analysis plot of NPL nonparametric microbial interdependence test (NMIT) distances at the genus level for samples from all treatments. Individual cones (AC cohorts) or spheres (all other cohorts) represent the longitudinal change in the microbial composition of each chinchilla (n = 27) across the experimental period. Differences in NMIT distances between treatments were analyzed in QIIME 2.0 by PERMANOVA with 999 randomizations of the data (P < 0.05) with P values displayed on respective graphs. Cohorts are colored according to treatment as denoted in the legend.
Fecal alpha diversity
Alpha diversity, a measure of microbiome composition within an individual sample, was assessed in each chinchilla across the sampled time points with measures of evenness, richness, Faith phylogenetic diversity (Faith PD), and Shannon diversity (Fig. 6). Omnibus test differences were analyzed with Kruskal–Wallis tests at each time point to determine whether any of the nine cohorts differed at a given time point. Pairwise comparisons between cohorts were assessed with Kruskal–Wallis tests and Benjamini–Hochberg correction for multiple testing to determine which specific cohorts differed from one another at each time point. Finally, pairwise comparisons were made within each cohort to assess whether alpha diversity within an individual cohort at 2DPT, 5DPT, 7DPT, or 9DPT differed from that same cohort's baseline value. All alpha diversity pairwise comparisons are reported as q-values (significant where q < 0.20) and are detailed in Supplementary File 3.
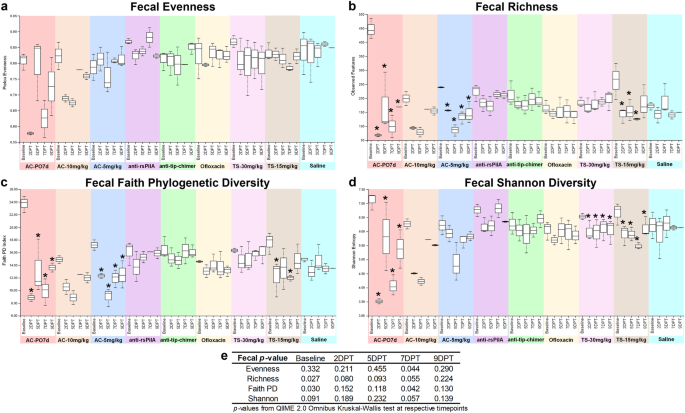
Measures of alpha diversity within fecal samples based on a evenness, b richness, c Faith phylogenetic diversity, and d Shannon diversity. e Omnibus tests for differences between any of the nine cohorts at each time point were performed with the Kruskal–Wallis tests in QIIME 2.0 (significant where P < 0.05). Values are represented as box and whisker plots where boxes denote interquartile range and upper and lower whiskers denote minimum and maximum, respectively. Each plot is organized by time point within the treatment. Asterisks denote significant pairwise comparisons (q < 0.20) between posttreatment time points and respective baseline values within individual cohorts. Sample sizes are reported in Table 1.
Omnibus test differences at baseline were observed for baseline fecal alpha richness (P = 0.027) and Faith PD (P = 0.030; Fig. 6e), however, differences more likely to be attributed to treatment were noted for 7DPT evenness (P = 0.044) and Faith PD (P = 0.042; Fig. 6e). Excitingly, alpha diversity in mAb-treated samples remained relatively consistent through 9DPT and exhibited minimal changes (Fig. 6a–d). Differences in pairwise comparisons between cohorts at posttreatment time points were only observed for fecal evenness and Shannon diversity at 7DPT (q < 0.20; Supplementary File 3) and were significantly lower in AC-PO7d relative to all cohorts except AC-10 mg/kg. Notably, when compared against saline, neither monoclonal antibody significantly differed.
It is not uncommon to observe variations in microbiome composition across healthy individuals of any species; however, due to baseline differences observed here that could potentially confound comparisons of treatment-mediated effects across cohorts, pairwise comparisons of alpha diversity were performed within each cohort for additional statistical perspective. Within-cohort comparisons allowed a more direct assessment of independent treatment effects and microbiome changes respective to that cohort's baseline. Comprehensive fecal pairwise comparisons between all combinations of sampling time points within a cohort are available in Supplementary Table 1, and significant pairwise comparisons made specifically between a cohort's posttreatment time points and respective baseline are represented as asterisks on fecal alpha diversity figures (Fig. 6a–d). Treatment-associated decreases in alpha diversity most consistently replicated in AC-PO7d, which had significantly lower (q < 0.20) fecal richness, Faith PD, and Shannon diversity at all time points relative to baseline (Fig. 6b–d). The same reduction (q < 0.20) in fecal alpha diversity across all time points relative to baseline was observed in AC-5 mg/kg richness and Faith PD (Fig. 6b, c), and in Shannon Diversity for both TS cohorts (Fig. 6d). In addition, relative to baseline, TS-15 mg/kg exhibited decreases (q < 0.20) in richness (2DPT, 5DPT, and 7DPT; Fig. 6b) and Faith PD (2DPT and 7DPT; Fig. 6c). These results demonstrated TT delivery of AC and TS could disrupt the gut microbiome as observed with oral AC, despite the more distant and somewhat less direct path between the middle ear and the gut. Moreover, mAb cohorts remained statistically unchanged relative to respective baselines for all fecal alpha diversity metrics.
Pairwise-differences, which measure longitudinal changes in alpha diversity, revealed a significant omnibus test difference for fecal richness over the baseline-5DPT interval (P = 0.039; Supplementary File 2). While direct pairwise comparisons between cohorts were insignificant over this interval (q > 0.20), an average of 274 features in AC-PO7d, 120 features in AC-10 mg/kg, 162 features in AC-5 mg/kg, and 107 features in TS-15 mg/kg were lost relative to respective baseline richness versus an average relative loss of less than 68 features in all remaining cohorts including mAbs (Supplementary File 2). Collectively, fecal alpha diversity results indicate that oral and TT delivery of AC have significantly disruptive effects on the gut microbiome. In addition, TS, an OM-relevant antibiotic administered to those with penicillin allergies, can also alter the fecal microbiome. As opposed to these standard-of-care antibiotics, neither mAb lead to changes in fecal alpha diversity.
Nasopharyngeal lavage alpha diversity
Alpha diversity in NPL samples was analyzed and is presented in a manner identical to that specified for fecal sample alpha diversity analysis above. Baseline differences in NPL alpha diversity were limited to richness (P = 0.016), and no omnibus test differences were observed at posttreatment time points. Pairwise comparisons between all cohorts are reported in Supplementary File 3. Comprehensive NPL pairwise comparisons between all combinations of sampling time points within a cohort are available in Supplementary Table 2, and significant pairwise comparisons made specifically between a cohort's posttreatment time points and respective baseline are re...
Comments
Post a Comment